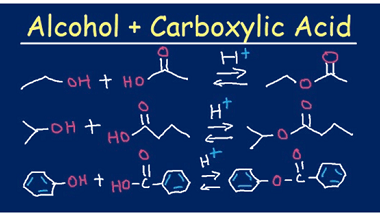
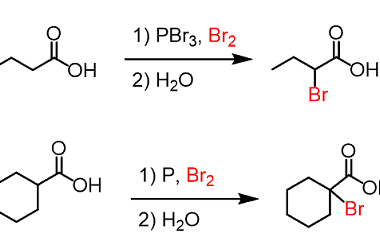
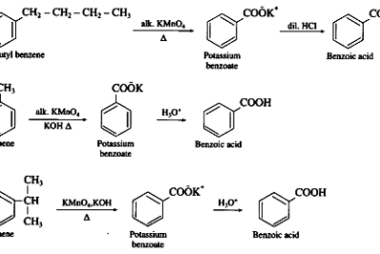
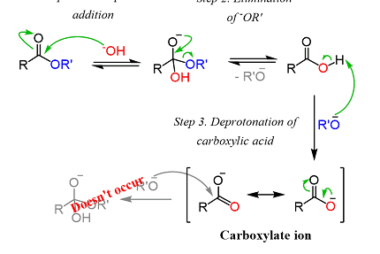
Acid chlorides
Acid chlorides are a class of organic compounds that contain a functional group consisting of a carbonyl group (C=O) bonded to a chlorine atom (Cl) and another functional group. They are also known as acyl chlorides or chloroformates. The general formula for an acid chloride is RCOCl, where R is an alkyl or aryl group.…