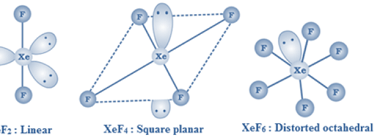
Group 18 Compounds of xenon with fluorine and oxygen
Xenon, a noble gas, can form compounds with fluorine and oxygen due to its large atomic size and availability of d-orbitals. Overall, the xenon-fluorine and xenon-oxygen compounds are quite reactive and can be dangerous to handle. However, they have important applications in industries such as electronics, materials science, and aerospace. What is Required p-Block Elements…