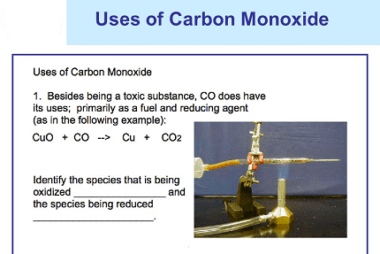
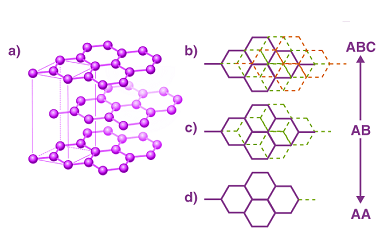
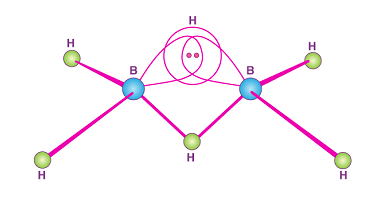
Group 14 Uses of carbon monoxide
Carbon monoxide (CO) is a colorless, odorless, and highly toxic gas. While it is primarily known for its harmful effects on human health, there are also some industrial and medical applications for carbon monoxide. Here are some of the uses of carbon monoxide: It is important to note that while carbon monoxide has some industrial…