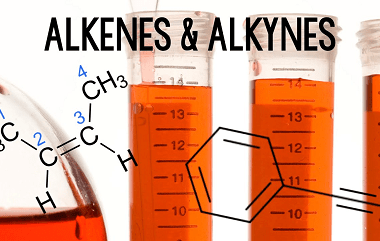
JEE (Main+Advance) e-Intermediate Course Alkenes and Alkynes
Alkenes and alkynes are unsaturated hydrocarbons that contain double and triple bonds, respectively. These compounds are important in organic chemistry because they undergo a variety of reactions that allow for the synthesis of more complex molecules. Some key topics that you might cover in the JEE (Main+Advance) e-Intermediate Course on Alkenes and Alkynes include: Overall,…