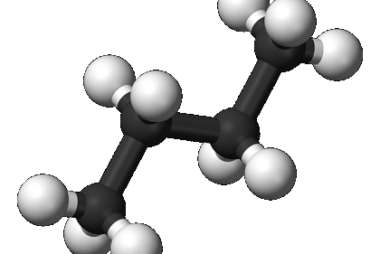
JEE (Main+Advance) Intermediate Course Alkenes and Alkynes
Sure, I can help you with that! Alkenes and alkynes are unsaturated hydrocarbons that contain at least one double or triple bond, respectively. Here are some important topics you should study for JEE (Main+Advanced) Intermediate Course Alkenes and Alkynes: Make sure to also practice solving problems and answering questions related to these topics to prepare…