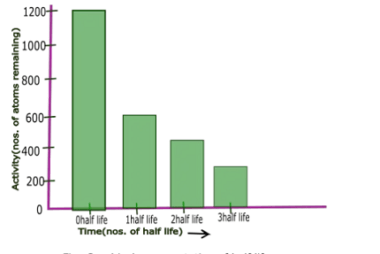
Half-life and Mean life
In the context of radioactivity, half-life and mean life are two important concepts that describe the decay of a radioactive substance over time. Half-life refers to the amount of time it takes for half of the original quantity of a radioactive substance to decay. For example, if you start with 100 grams of a substance…