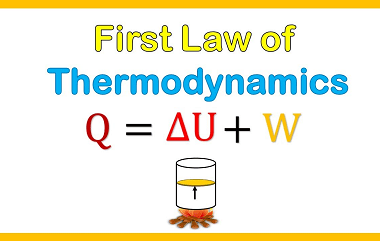
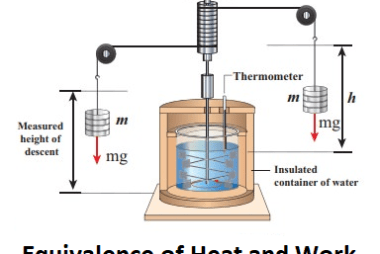
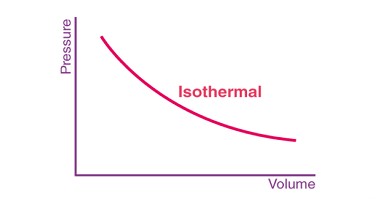
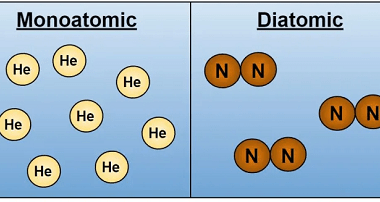
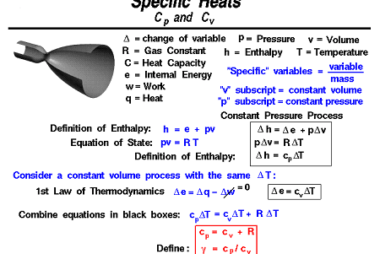
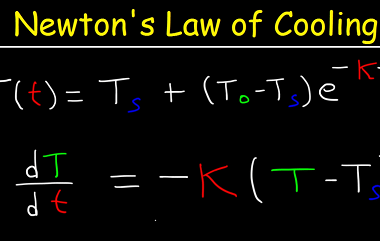
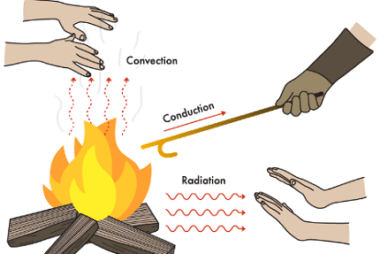
First law of thermodynamics
The First Law of Thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, but it can be transformed from one form to another or transferred from one system to another. In other words, the total energy of a closed system remains constant. This law is a…