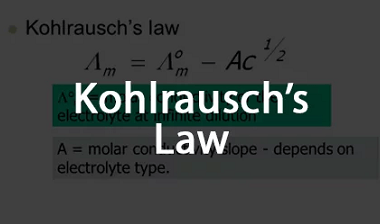
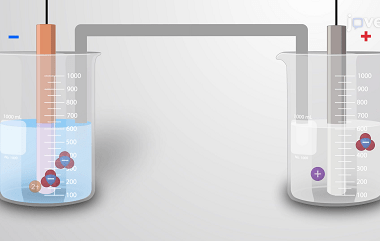
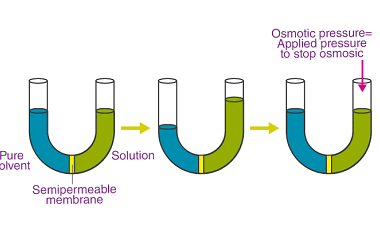
Advance Course AIIMS-SYLLABUS Chemistry syllabus Standard electrode potential
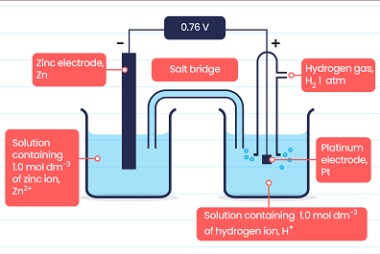
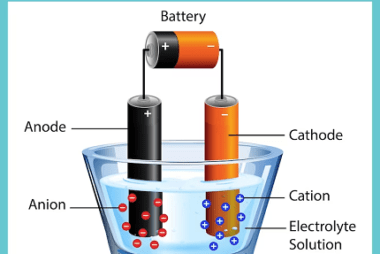
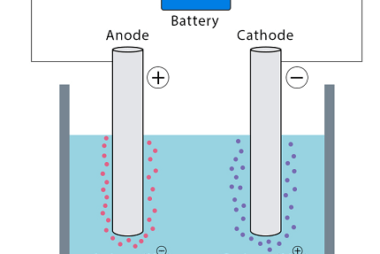
Standard electrode potential Standard electrode potential, also known as standard reduction potential, is a measure of the tendency of an electrode to gain or lose electrons compared to a standard reference electrode under standard conditions. It represents the voltage or potential difference between the electrode and the reference electrode. The standard reference electrode used is…