Advance Course AIIMS-SYLLABUS Chemistry syllabus Chemisorption
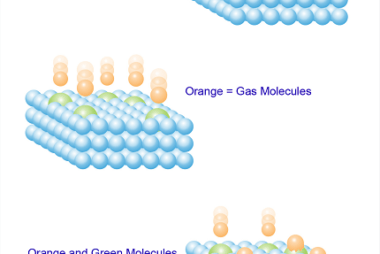
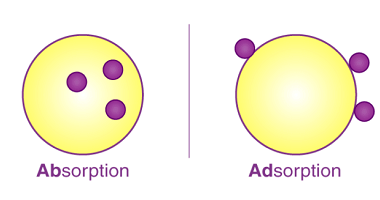
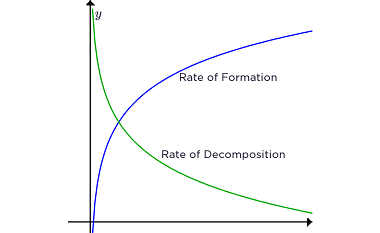
Chemisorption Chemisorption is a process of adsorption in which a chemical reaction occurs between the adsorbate (molecules or atoms being adsorbed) and the surface of the adsorbent (the material on which adsorption takes place). It involves the formation of chemical bonds between the adsorbate and adsorbent, resulting in the creation of a new compound or…