Classification of solids based on different binding forces such as molecular
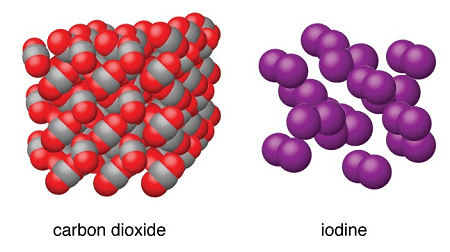
Solids can be classified based on different binding forces, including molecular forces. This classification is based on the nature of the intermolecular forces that hold the constituent particles together. Here are the main types of solids based on molecular binding forces:
- Molecular Solids: These solids are composed of discrete molecules held together by weak intermolecular forces such as van der Waals forces or hydrogen bonding. Examples include solid water (ice), organic compounds like sugar, and gases like carbon dioxide (dry ice).
- Ionic Solids: Ionic solids consist of a three-dimensional array of positively and negatively charged ions. The ions are held together by strong electrostatic forces. Examples include sodium chloride (table salt), calcium carbonate, and potassium nitrate.
- Covalent Network Solids: In covalent network solids, the constituent atoms are bonded together by a network of covalent bonds, forming a continuous three-dimensional structure. This results in a very strong and rigid solid. Examples include diamond (composed of carbon atoms) and quartz (composed of silicon and oxygen atoms).
- Metallic Solids: Metallic solids are composed of closely packed positive metal ions immersed in a “sea” of delocalized electrons. The metallic bonds, which are a result of the attraction between positive ions and delocalized electrons, give metals their characteristic properties such as high electrical and thermal conductivity. Examples include copper, iron, and gold.
It’s important to note that some solids may exhibit a combination of different bonding forces.
What is Required Chemistry syllabus Classification of solids based on different binding forces such as molecular
The required chemistry syllabus for the classification of solids based on different binding forces, such as molecular forces, typically includes the following topics:
- Molecular Solids:
- Definition and characteristics of molecular solids.
- Intermolecular forces in molecular solids, such as van der Waals forces and hydrogen bonding.
- Examples and properties of molecular solids, including their melting points, solubility, and electrical conductivity.
- Ionic Solids:
- Definition and characteristics of ionic solids.
- Formation and properties of ions.
- Ionic bonding and the role of electrostatic forces in holding ions together.
- Examples and properties of common ionic solids, including their crystal structures, melting points, solubility, and electrical conductivity.
- Covalent Network Solids:
- Definition and characteristics of covalent network solids.
- Covalent bonding and its role in forming a continuous three-dimensional network.
- Examples and properties of covalent network solids, such as diamond and quartz.
- Comparison of covalent network solids with other types of solids.
- Metallic Solids:
- Definition and characteristics of metallic solids.
- Metallic bonding and the role of delocalized electrons in holding metal atoms together.
- Properties of metallic solids, including high electrical and thermal conductivity, malleability, and ductility.
- Examples and properties of common metallic solids.
It’s important to consult the specific syllabus provided by the educational institution or exam board to ensure comprehensive coverage of the classification of solids based on different binding forces, as syllabus requirements may vary.
When is Required Chemistry syllabus Classification of solids based on different binding forces such as molecular
The classification of solids based on different binding forces, such as molecular forces, is typically covered in the chemistry syllabus at the high school level or in introductory college-level chemistry courses. The exact timing may vary depending on the educational institution and curriculum structure. In most cases, this topic is covered as part of a broader unit on the properties of solids and bonding in chemistry. It is important to consult the specific syllabus or curriculum provided by the educational institution to determine the exact timing and depth of coverage for the classification of solids based on different binding forces.
Where is Required Chemistry syllabus Classification of solids based on different binding forces such as molecular
The required chemistry syllabus for the classification of solids based on different binding forces, such as molecular forces, is typically found in the curriculum of chemistry courses. It is commonly covered in high school chemistry courses, as well as introductory college-level chemistry courses.
In high school, this topic may be part of the curriculum for courses such as General Chemistry or Advanced Placement (AP) Chemistry. The syllabus or curriculum document for these courses will outline the specific topics and concepts to be covered, including the classification of solids based on different binding forces.
At the college level, this topic is often included in introductory chemistry courses, such as College Chemistry or Principles of Chemistry. The syllabus provided by the college or university will specify the content covered in the course, including the classification of solids based on different binding forces.
It’s important to consult the specific syllabus or curriculum document provided by the educational institution to understand where the classification of solids based on different binding forces fits within the overall course structure and timeline.
How is Required Chemistry syllabus Classification of solids based on different binding forces such as molecular
The required chemistry syllabus for the classification of solids based on different binding forces, such as molecular forces, typically involves the following approach:
- Introduction: The topic begins with an introduction to solids and their characteristics. This includes defining solids and explaining their properties, such as definite shape and volume.
- Types of Solids: The syllabus will cover the different types of solids, including molecular solids, ionic solids, covalent network solids, and metallic solids. Each type will be described in terms of its composition, structure, and bonding.
- Molecular Solids: The syllabus will focus on molecular solids, emphasizing the intermolecular forces that hold the molecules together. This includes discussing van der Waals forces, dipole-dipole interactions, and hydrogen bonding. Examples of molecular solids and their properties, such as solubility and melting point, may also be covered.
- Ionic Solids: The syllabus will cover ionic solids, highlighting the strong electrostatic forces that exist between positively and negatively charged ions. Concepts such as lattice energy, crystal structures, and the properties of ionic compounds will be addressed. Examples of common ionic solids may be included.
- Covalent Network Solids: The syllabus will cover covalent network solids, focusing on the continuous three-dimensional network of covalent bonds. Examples such as diamond and quartz will be discussed, along with their properties and unique characteristics.
- Metallic Solids: The syllabus will cover metallic solids, emphasizing metallic bonding and the presence of delocalized electrons. Properties such as high electrical and thermal conductivity, malleability, and ductility will be explored. Examples of common metallic solids may also be included.
The syllabus may include additional subtopics or variations depending on the educational institution or specific curriculum. It’s essential to refer to the provided syllabus or curriculum document for a comprehensive understanding of the content and the specific approach taken in the classification of solids based on different binding forces.
Structures of Chemistry syllabus Classification of solids based on different binding forces such as molecular
The structure of the chemistry syllabus for the classification of solids based on different binding forces, such as molecular forces, can vary depending on the educational institution and curriculum. However, here is a general structure that can be followed:
- Introduction to Solids
- Definition and characteristics of solids.
- Differences between solids, liquids, and gases.
- Importance of studying solid-state chemistry.
- Molecular Solids
- Definition and properties of molecular solids.
- Intermolecular forces: van der Waals forces, dipole-dipole interactions, and hydrogen bonding.
- Examples of molecular solids and their properties.
- Melting points, solubility, and electrical conductivity of molecular solids.
- Ionic Solids
- Definition and properties of ionic solids.
- Formation and properties of ions.
- Ionic bonding: electrostatic forces between positive and negative ions.
- Crystal structures of ionic solids.
- Examples of common ionic solids and their properties.
- Melting points, solubility, and electrical conductivity of ionic solids.
- Covalent Network Solids
- Definition and properties of covalent network solids.
- Covalent bonding: formation of a continuous network of covalent bonds.
- Crystal structures of covalent network solids.
- Examples of covalent network solids (e.g., diamond, quartz) and their properties.
- Comparison with other types of solids.
- Metallic Solids
- Definition and properties of metallic solids.
- Metallic bonding: delocalized electrons and positive metal ions.
- Crystal structures of metallic solids.
- Examples of metallic solids and their properties.
- High electrical and thermal conductivity, malleability, and ductility of metals.
It’s important to note that this structure is a general guideline and may be adapted or modified based on the specific syllabus provided by the educational institution. The syllabus will provide a more detailed outline of the topics to be covered, subtopics, and any additional concepts or examples that may be included.
Case Study on Chemistry syllabus Classification of solids based on different binding forces such as molecular
Case Study: Classification of Solids Based on Different Binding Forces – Molecular Solids
Introduction: In this case study, we will explore the classification of solids based on different binding forces, focusing specifically on molecular solids. Molecular solids are composed of discrete molecules held together by weak intermolecular forces such as van der Waals forces or hydrogen bonding. We will examine the properties, examples, and applications of molecular solids to understand their significance in various fields.
Properties of Molecular Solids:
- Intermolecular Forces: Molecular solids are held together by intermolecular forces, which are relatively weak compared to chemical bonds. These forces include van der Waals forces, dipole-dipole interactions, and hydrogen bonding.
- Melting Points: Molecular solids generally have lower melting points compared to other types of solids due to the weaker intermolecular forces.
- Solubility: The solubility of molecular solids depends on the nature of the intermolecular forces and the polarity of the solvent. Polar molecular solids tend to be soluble in polar solvents, while nonpolar molecular solids dissolve better in nonpolar solvents.
- Electrical Conductivity: Molecular solids are usually poor conductors of electricity since their constituent molecules do not have free charge carriers.
Examples of Molecular Solids:
- Solid Water (Ice): Ice is a well-known example of a molecular solid. It consists of water molecules held together by hydrogen bonding. It has a specific crystalline structure and displays unique properties, such as expansion upon freezing.
- Organic Compounds: Many organic compounds form molecular solids. For example, sugar (sucrose) crystallizes to form a molecular solid held together by a network of hydrogen bonds. Other examples include organic dyes, pharmaceuticals, and natural products.
- Dry Ice (Solid Carbon Dioxide): Solid carbon dioxide, commonly known as dry ice, is composed of carbon dioxide molecules held together by van der Waals forces. It is widely used for cooling purposes, transportation of perishable goods, and in special effects.
Applications of Molecular Solids:
- Pharmaceutical Industry: Molecular solids play a crucial role in the pharmaceutical industry. Many drugs are formulated as solid dosage forms, such as tablets or capsules, utilizing the properties of molecular solids for stability, controlled release, and ease of administration.
- Materials Science: Molecular solids are employed in materials science for their unique properties. For example, certain molecular solids exhibit piezoelectric properties, making them useful in sensors and actuators.
- Food and Beverage Industry: Molecular solids like sugar are extensively used in the food and beverage industry for their sweetening properties. They contribute to the taste, texture, and stability of various products.
Conclusion: The classification of solids based on different binding forces, such as molecular forces, is important for understanding the properties and applications of various solid materials. Molecular solids, characterized by weak intermolecular forces, have distinct properties and find applications in fields ranging from pharmaceuticals to materials science. Exploring the classification of solids and their specific examples provides valuable insights into the diverse nature of solids and their impact on different industries.
White paper on Chemistry syllabus Classification of solids based on different binding forces such as molecular
Title: Classification of Solids Based on Different Binding Forces: A Comprehensive White Paper
Abstract: This white paper provides a comprehensive overview of the classification of solids based on different binding forces, with a specific focus on molecular solids. The paper explores the properties, examples, and applications of molecular solids in various fields. Understanding the diverse nature of solids and their binding forces is crucial for advancing materials science, pharmaceuticals, and other industries that heavily rely on solid-state chemistry. This white paper aims to provide valuable insights and serve as a reference for researchers, educators, and professionals working in related fields.
- Introduction
- Importance of understanding the classification of solids based on binding forces.
- Overview of the different types of solids and their binding forces.
- Focus on molecular solids and their unique characteristics.
- Molecular Solids: Properties and Characteristics
- Definition and key features of molecular solids.
- Intermolecular forces and their significance in molecular solids.
- Melting points, solubility, and electrical conductivity of molecular solids.
- Intermolecular Forces in Molecular Solids
- Van der Waals forces: Dispersion forces and dipole-dipole interactions.
- Hydrogen bonding and its role in molecular solids.
- Other intermolecular forces present in specific molecular solids.
- Examples of Molecular Solids
- Solid Water (Ice): Structure, hydrogen bonding, and properties.
- Organic Compounds: Sugar, dyes, pharmaceuticals, and natural products.
- Dry Ice (Solid Carbon Dioxide): Formation, structure, and applications.
- Applications of Molecular Solids
- Pharmaceutical industry: Solid dosage forms, stability, and controlled release.
- Materials science: Piezoelectric properties and their applications.
- Food and beverage industry: Sweetening agents, stability, and texture enhancement.
- Experimental Techniques and Analysis
- Techniques for studying molecular solids, including X-ray crystallography and spectroscopy.
- Computational methods for predicting and analyzing intermolecular forces.
- Role of molecular modeling and simulations in understanding molecular solids.
- Future Perspectives and Challenges
- Advances in the design and synthesis of molecular solids.
- Exploration of new intermolecular forces and their impact on solid-state chemistry.
- Challenges and opportunities in the field of molecular solids research.
- Conclusion
- Recap of the key points discussed.
- Importance of continued research and exploration in the field.
- Potential for molecular solids in driving technological advancements.
This white paper serves as a comprehensive resource on the classification of solids based on different binding forces, particularly focusing on molecular solids. By providing an in-depth understanding of the properties, examples, and applications of molecular solids, this white paper aims to contribute to the advancement of solid-state chemistry and its various applications in diverse industries.