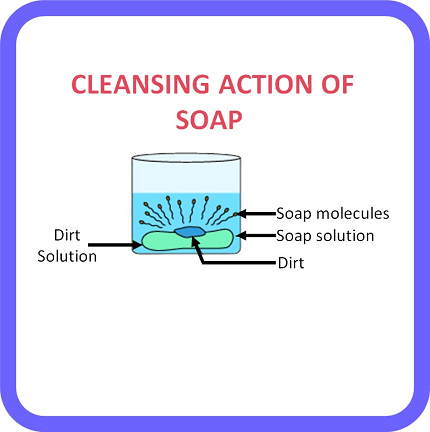
The term “cleansing action” can refer to a few different things depending on the context. Here are a few possible meanings:
- In the context of skincare or beauty products, “cleansing action” refers to the ability of a product to remove dirt, oil, and impurities from the skin. Cleansers, facial scrubs, and exfoliating products are all examples of products that might have a “cleansing action.”
- In the context of nutrition, “cleansing action” might refer to the ability of certain foods or supplements to promote detoxification or elimination of waste from the body. For example, some people believe that drinking lemon water or taking activated charcoal supplements can have a “cleansing action” on the digestive system.
- In a more general sense, “cleansing action” could refer to any action taken to remove something unwanted or harmful from a system or environment. For example, a company might take steps to “cleanse” its corporate culture of toxic behavior, or a city might undertake a “cleansing action” to rid its streets of litter and debris.
What is Required Chemistry in Everyday Life Cleansing action
The required chemistry for cleansing action in everyday life depends on the specific cleansing product or process being used. However, there are a few general principles of chemistry that are important to understand in order to appreciate how cleansing products work:
- Surfactants: Many cleansing products, such as soaps and detergents, contain surfactants. Surfactants are compounds that reduce the surface tension between two liquids or between a liquid and a solid. This allows the cleansing product to more easily mix with water and to penetrate and lift dirt, oil, and other impurities from surfaces.
- pH: The pH of a cleansing product can also be important. For example, acidic products (with a pH below 7) are often used to remove hard water stains, while alkaline products (with a pH above 7) are often used to remove grease and oil. Understanding the pH of a cleansing product can help ensure that it is used correctly and safely.
- Chemical reactions: Some cleansing products work by causing chemical reactions with the substances they are intended to remove. For example, bleach is a common cleaning agent that works by reacting with the pigments in stains to break them down and make them easier to remove.
Overall, understanding the chemistry behind cleansing products can help us make informed choices about which products to use for different tasks, as well as how to use them safely and effectively.
When is Required Chemistry in Everyday Life Cleansing action
The required chemistry for cleansing action in everyday life is present in a wide range of products and processes that are used to clean and maintain our homes, bodies, and environments. Here are some examples:
- Household cleaning products: Chemistry is essential for the formulation of household cleaning products such as detergents, surface cleaners, and disinfectants. These products contain various chemical compounds such as surfactants, enzymes, acids, and alkalis, which help to dissolve, emulsify, or neutralize different types of dirt, stains, and microorganisms.
- Personal care products: The chemistry of cleansing is also important in personal care products such as soaps, shampoos, toothpaste, and facial cleansers. These products often contain surfactants and other ingredients that help to remove oil, sweat, dead skin cells, and other impurities from the skin or hair.
- Water treatment: Chemistry plays a crucial role in the treatment of water for household and industrial use. Water treatment processes involve various chemical reactions such as coagulation, flocculation, sedimentation, filtration, and disinfection, which help to remove contaminants and make water safe for consumption.
- Environmental cleanup: Chemistry is also essential in environmental cleanup efforts such as oil spills, chemical spills, and hazardous waste remediation. Chemical methods such as absorption, adsorption, oxidation, and biodegradation are often used to contain, neutralize, or remove harmful substances from the environment.
Overall, the required chemistry for cleansing action is present in a wide range of products and processes that are essential for maintaining a clean and healthy environment.
Where is Required Chemistry in Everyday Life Cleansing action
The required chemistry for cleansing action in everyday life can be found in a variety of products and processes used to clean and maintain our homes, bodies, and surroundings. Here are some examples:
- Cleaning products: Household cleaning products such as detergents, soaps, and disinfectants contain various chemical compounds such as surfactants, enzymes, acids, and alkalis, which help to dissolve, emulsify, or neutralize different types of dirt, stains, and microorganisms.
- Personal care products: The chemistry of cleansing is also important in personal care products such as shampoos, toothpaste, and facial cleansers. These products often contain surfactants and other ingredients that help to remove oil, sweat, dead skin cells, and other impurities from the skin or hair.
- Water treatment: Chemistry plays a crucial role in the treatment of water for household and industrial use. Water treatment processes involve various chemical reactions such as coagulation, flocculation, sedimentation, filtration, and disinfection, which help to remove contaminants and make water safe for consumption.
- Environmental cleanup: The required chemistry for cleansing action is also important in environmental cleanup efforts such as oil spills, chemical spills, and hazardous waste remediation. Chemical methods such as absorption, adsorption, oxidation, and biodegradation are often used to contain, neutralize, or remove harmful substances from the environment.
Overall, the required chemistry for cleansing action is present in many aspects of our daily lives, from the cleaning products we use to the water we drink and the environment we live in.
How is Required Chemistry in Everyday Life Cleansing action
The required chemistry for cleansing action in everyday life works in different ways depending on the specific product or process being used. However, there are some general principles of chemistry that are important to understand in order to appreciate how cleansing products work:
- Surfactants: Many cleansing products contain surfactants, which are compounds that lower the surface tension between two liquids or between a liquid and a solid. This allows the cleansing product to more easily mix with water and to penetrate and lift dirt, oil, and other impurities from surfaces.
- Acids and alkalis: Acids and alkalis are often used in cleansing products to neutralize or dissolve different types of substances. For example, acids are commonly used to remove hard water stains and alkalis are used to remove grease and oil.
- Oxidation: Some cleansing products work by causing oxidation reactions, which break down and remove stains or other organic materials. For example, bleach is a common cleaning agent that works by oxidizing the pigments in stains to break them down and make them easier to remove.
- Enzymes: Enzymes are biological catalysts that can break down complex molecules into simpler forms. Some cleansing products, such as laundry detergents and stain removers, contain enzymes that can break down and remove specific types of stains or soils.
Overall, the required chemistry for cleansing action in everyday life works through a variety of mechanisms that help to remove dirt, stains, and other impurities from surfaces, materials, and environments. Understanding the chemistry behind cleansing products can help us choose the most effective and safe products for different cleaning tasks.
Structures of Chemistry in Everyday Life Cleansing action
The structures of chemistry in everyday life cleansing action are varied and depend on the specific product or process being used. However, here are some examples of chemical structures commonly found in cleansing products:
- Surfactants: Surfactants are compounds that contain a hydrophobic (water-repelling) and a hydrophilic (water-attracting) portion. This dual nature allows surfactants to lower the surface tension of water and to dissolve and remove dirt, oil, and other impurities from surfaces. Examples of surfactants used in cleansing products include sodium lauryl sulfate, sodium laureth sulfate, and cocoamidopropyl betaine.
- Acids and alkalis: Acids and alkalis are compounds that can donate or accept protons, respectively. This property allows them to neutralize or dissolve different types of substances. Examples of acids used in cleansing products include citric acid, acetic acid, and hydrochloric acid, while examples of alkalis include sodium hydroxide, potassium hydroxide, and ammonia.
- Enzymes: Enzymes are biological catalysts that can break down complex molecules into simpler forms. Some cleansing products, such as laundry detergents and stain removers, contain enzymes that can break down and remove specific types of stains or soils. Examples of enzymes used in cleansing products include proteases, amylases, and lipases.
- Oxidizing agents: Oxidizing agents are compounds that can donate oxygen or remove electrons from other compounds. This property allows them to break down and remove stains or other organic materials. Examples of oxidizing agents used in cleansing products include hydrogen peroxide, sodium hypochlorite (bleach), and potassium permanganate.
Overall, the structures of chemistry in everyday life cleansing action are diverse and complex, and their properties and functions depend on their specific chemical composition and interactions with other substances.
Case Study on Chemistry in Everyday Life Cleansing action
Case Study: Chemistry in Everyday Life Cleansing Action – The Science behind Laundry Detergent
Laundry detergent is a common household cleaning product that contains a variety of chemical compounds designed to remove dirt, stains, and odors from clothing and other fabrics. The chemistry of laundry detergent involves several different types of chemical reactions and interactions that work together to achieve effective cleaning.
The main components of laundry detergent include surfactants, enzymes, builders, and other additives. Here’s how each of these components works:
- Surfactants: Laundry detergents contain surfactants, which are compounds that lower the surface tension of water and help to penetrate and remove dirt and stains from fabrics. Surfactants contain both hydrophilic and hydrophobic regions, which allow them to attach to both water and dirt, and then lift the dirt away from the fabric. Examples of surfactants used in laundry detergents include sodium lauryl sulfate and sodium laureth sulfate.
- Enzymes: Enzymes are biological catalysts that can break down complex molecules into simpler forms. Laundry detergents often contain enzymes such as proteases, amylases, and lipases, which help to break down and remove specific types of stains and soils. Proteases, for example, can break down protein-based stains such as blood and grass, while amylases can break down starch-based stains such as food and mud.
- Builders: Builders are chemical compounds that help to soften hard water and to boost the cleaning power of detergents. Hard water contains high levels of calcium and magnesium ions, which can interfere with the cleaning process and cause soap scum and other deposits to form on fabrics. Builders such as sodium tripolyphosphate and zeolites can bind to these ions and prevent them from interfering with the cleaning process.
- Other additives: Laundry detergents may also contain other additives such as optical brighteners, fragrances, and anti-redeposition agents. Optical brighteners are compounds that absorb ultraviolet light and re-emit it as visible light, making fabrics appear brighter and whiter. Fragrances are added to provide a pleasant scent to freshly washed clothing. Anti-redeposition agents are compounds that prevent dirt and other particles from reattaching to fabrics during the washing cycle.
In summary, the chemistry of laundry detergent is a complex and multifaceted process that involves the interaction of several different chemical compounds and reactions. By understanding the science behind laundry detergent, consumers can make informed choices about the products they use to clean their clothes and other fabrics.
White paper on Chemistry in Everyday Life Cleansing action
White Paper: Chemistry in Everyday Life Cleansing Action – The Role of Chemicals in Modern Cleaning Products
Introduction:
Cleansing action is a critical aspect of modern life. Every day, we rely on a variety of cleaning products to remove dirt, stains, and odors from our homes, workplaces, and personal belongings. From laundry detergent to surface cleaners, these products use a range of chemical compounds to achieve effective cleaning. This white paper explores the role of chemicals in everyday life cleansing action, with a focus on the science behind modern cleaning products.
Chemical Compounds in Cleaning Products:
Cleaning products contain a variety of chemical compounds designed to remove different types of soils and stains. The main types of chemicals used in cleaning products include:
- Surfactants: Surfactants are compounds that reduce the surface tension of water, allowing it to penetrate and remove dirt and stains from surfaces. Surfactants contain both hydrophilic and hydrophobic regions, which allow them to attach to both water and dirt, and then lift the dirt away from the surface. Examples of surfactants used in cleaning products include sodium lauryl sulfate, sodium laureth sulfate, and cocoamidopropyl betaine.
- Acids and Alkalis: Acids and alkalis are compounds that can donate or accept protons, respectively. This property allows them to neutralize or dissolve different types of substances. Examples of acids used in cleaning products include citric acid, acetic acid, and hydrochloric acid, while examples of alkalis include sodium hydroxide, potassium hydroxide, and ammonia.
- Enzymes: Enzymes are biological catalysts that can break down complex molecules into simpler forms. Some cleaning products, such as laundry detergents and stain removers, contain enzymes that can break down and remove specific types of stains or soils. Examples of enzymes used in cleaning products include proteases, amylases, and lipases.
- Oxidizing Agents: Oxidizing agents are compounds that can donate oxygen or remove electrons from other compounds. This property allows them to break down and remove stains or other organic materials. Examples of oxidizing agents used in cleaning products include hydrogen peroxide, sodium hypochlorite (bleach), and potassium permanganate.
Chemical Interactions in Cleaning Products:
The effectiveness of cleaning products depends not only on the chemical compounds they contain, but also on the interactions between these compounds. For example, surfactants may interact with enzymes to enhance their effectiveness in breaking down specific types of soils and stains. Similarly, the addition of builders such as sodium tripolyphosphate or zeolites can help to soften hard water and improve the overall cleaning performance of the product.
Environmental Considerations:
While cleaning products are essential for maintaining a clean and healthy environment, it is important to consider their potential impact on the environment. Many cleaning products contain chemicals that can be harmful to aquatic life, and may contribute to water pollution. To address these concerns, many manufacturers have developed environmentally-friendly cleaning products that use biodegradable or renewable ingredients, and are designed to be safe for both people and the environment.
Conclusion:
Chemistry plays a crucial role in everyday life cleansing action. The chemical compounds and interactions in modern cleaning products enable us to maintain clean and healthy living environments. By understanding the science behind these products, consumers can make informed choices about the cleaning products they use, while also considering their potential impact on the environment.
