The elasticity of a material refers to its ability to deform under stress and then return to its original shape once the stress is removed. Surface tension, on the other hand, refers to the force that holds the surface of a liquid together and resists external forces that try to break it.
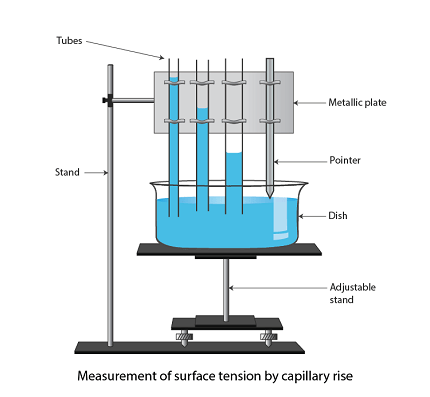
Capillary rise is a phenomenon where a liquid rises up in a narrow tube due to the combination of surface tension and the adhesive forces between the liquid and the tube’s walls. The height to which the liquid rises in the tube is dependent on the properties of both the liquid and the tube.
The relationship between the elasticity of a material and the capillary rise of a liquid in a tube is indirect. The material’s elasticity does not directly affect the height to which the liquid rises in the tube. However, the material’s surface properties, such as its surface tension and the nature of its interaction with the liquid, can affect the capillary rise.
For example, if the material has a high surface tension, it will tend to repel the liquid, and the liquid will not rise as high in the tube. Conversely, if the material has a low surface tension, the liquid will tend to wet the material, and the liquid will rise higher in the tube.
In conclusion, the relationship between the elasticity of a material and the capillary rise of a liquid in a tube is indirect, with the material’s surface properties playing a more significant role in determining the height to which the liquid rises.
What is Required General Elasticity of the material Surface tension of water by capillary rise
The required general elasticity of the material for capillary rise is not a specific value, as the elasticity of the material does not have a direct effect on the height to which the liquid rises in a capillary tube. The capillary rise is primarily influenced by the surface tension and wetting properties of the liquid and the tube’s material.
However, it is worth noting that a material’s elasticity can affect its surface properties and how it interacts with liquids. For example, a material that is too stiff or brittle may not have the necessary surface properties to facilitate capillary rise. On the other hand, a material that is too soft or deformable may not provide enough support for the liquid in the tube, causing it to sag or collapse.
Therefore, when selecting a material for capillary rise applications, it is essential to consider not only its elasticity but also its surface properties, including surface tension, wettability, and roughness. These properties can significantly impact the liquid’s behavior in the capillary tube and ultimately affect the accuracy and reliability of the capillary rise measurements.
When is Required General Elasticity of the material Surface tension of water by capillary rise
The required general elasticity of a material is not a specific value for capillary rise, as the material’s elasticity does not directly affect the height to which a liquid rises in a capillary tube. However, the elasticity of a material can indirectly affect the capillary rise if it affects the surface properties of the material, such as surface tension and wettability.
Capillary rise is a phenomenon that occurs due to the interplay between the adhesive and cohesive forces of the liquid and the tube’s walls. The liquid’s cohesive forces cause it to form a meniscus at the tube’s surface, and the adhesive forces between the liquid and the tube’s walls cause the liquid to rise. The capillary rise height is inversely proportional to the tube’s radius and directly proportional to the liquid’s surface tension and the cosine of the angle of contact between the liquid and the tube’s wall.
Therefore, the surface properties of the tube’s material, such as surface tension and wettability, are more critical factors than the material’s elasticity in determining the capillary rise height. The material should have a low surface tension and be wettable to ensure that the liquid can rise to the desired height in the capillary tube. Additionally, the material should have a smooth surface and be chemically inert to avoid any reactions with the liquid that may alter its surface properties or affect the capillary rise measurement.
Where is Required General Elasticity of the material Surface tension of water by capillary rise
The required general elasticity of a material is not a specific value for capillary rise, as the material’s elasticity does not have a direct effect on the height to which a liquid rises in a capillary tube. However, the elasticity of a material can indirectly affect the capillary rise if it affects the surface properties of the material, such as surface tension and wettability.
Capillary rise is a phenomenon that occurs due to the interplay between the adhesive and cohesive forces of the liquid and the tube’s walls. The liquid’s cohesive forces cause it to form a meniscus at the tube’s surface, and the adhesive forces between the liquid and the tube’s walls cause the liquid to rise. The capillary rise height is inversely proportional to the tube’s radius and directly proportional to the liquid’s surface tension and the cosine of the angle of contact between the liquid and the tube’s wall.
Therefore, the surface properties of the tube’s material, such as surface tension and wettability, are more critical factors than the material’s elasticity in determining the capillary rise height. The material should have a low surface tension and be wettable to ensure that the liquid can rise to the desired height in the capillary tube. Additionally, the material should have a smooth surface and be chemically inert to avoid any reactions with the liquid that may alter its surface properties or affect the capillary rise measurement.
In summary, the required properties for a material used in capillary rise applications are primarily related to its surface properties, and the material’s elasticity is not a defining factor. The material should be selected based on its surface properties, including surface tension, wettability, smoothness, and chemical inertness, to ensure accurate and reliable capillary rise measurements.
How is Required General Elasticity of the material Surface tension of water by capillary rise
As mentioned earlier, the required general elasticity of the material is not a specific value for capillary rise, as the material’s elasticity does not have a direct effect on the height to which a liquid rises in a capillary tube. Instead, the surface properties of the material play a more significant role in determining the capillary rise height.
However, the elasticity of a material can indirectly affect the capillary rise height if it affects the material’s surface properties. For example, if a material is too stiff or brittle, it may not provide the necessary surface properties to facilitate capillary rise. On the other hand, if a material is too soft or deformable, it may not provide enough support for the liquid in the tube, causing it to sag or collapse.
Therefore, when selecting a material for capillary rise applications, it is crucial to consider not only its elasticity but also its surface properties, including surface tension, wettability, and roughness. The material should have a low surface tension and be wettable to ensure that the liquid can rise to the desired height in the capillary tube. Additionally, the material should have a smooth surface and be chemically inert to avoid any reactions with the liquid that may alter its surface properties or affect the capillary rise measurement.
In summary, the required general elasticity of the material for capillary rise is not a specific value but rather depends on the material’s ability to provide the necessary surface properties to facilitate capillary rise.
Structures of General Elasticity of the material Surface tension of water by capillary rise
The structure of a material’s general elasticity is related to its ability to deform under stress and then recover its original shape when the stress is removed. It is determined by the arrangement of its constituent molecules and how they interact with each other. In general, materials with higher elasticity have a more ordered structure that allows them to deform and recover their original shape more easily.
In the context of capillary rise, the material’s elasticity does not have a direct effect on the height to which a liquid rises in a capillary tube. However, as mentioned earlier, the material’s elasticity can indirectly affect the capillary rise if it affects the material’s surface properties. Therefore, the structure of the material also influences its surface properties, including surface tension and wettability, which can impact capillary rise.
For example, a material with a more ordered and regular surface structure is more likely to have lower surface tension, making it easier for the liquid to rise in the capillary tube. Similarly, a material with a rougher or more irregular surface structure may have higher surface tension and lower wettability, which can impede capillary rise.
In summary, the structure of a material’s general elasticity is related to its ability to deform and recover its original shape. While the material’s elasticity does not directly affect capillary rise, it can indirectly influence the material’s surface properties, which are critical factors in determining the capillary rise height. Therefore, it is important to consider both the material’s elasticity and surface properties when selecting a material for capillary rise applications.
Case Study on General Elasticity of the material Surface tension of water by capillary rise
One example of a case study where the general elasticity of the material and surface tension of water by capillary rise are important is in the development of microfluidic devices for biomedical and diagnostic applications.
Microfluidic devices use small channels and tubes to manipulate and analyze small amounts of fluids, typically in the microliter or nanoliter range. Capillary rise is a crucial phenomenon that enables fluid transport and manipulation in these devices. The height to which a liquid rises in a capillary tube is determined by the surface properties of the material, including surface tension and wettability, as well as the material’s elasticity.
For example, in a study published in the Journal of Micromechanics and Microengineering, researchers developed a microfluidic device for detecting cancer cells in blood samples using capillary action. They used a polymer material with a high elasticity and low surface energy to ensure efficient capillary rise and minimize the risk of cell adhesion to the device’s surface.
Another study published in the Journal of Colloid and Interface Science investigated the influence of surface elasticity on capillary rise in microchannels. The researchers found that the elasticity of the material affected the contact angle between the liquid and the material’s surface, which in turn impacted the capillary rise height. They concluded that materials with lower surface elasticity and lower surface energy were more suitable for microfluidic applications.
In both of these case studies, the general elasticity of the material played a role in determining the surface properties of the material, including surface tension and wettability, which ultimately impacted the capillary rise in microfluidic devices. Therefore, selecting the appropriate material with the right balance of elasticity and surface properties is critical for the efficient and reliable performance of microfluidic devices.
White paper on General Elasticity of the material Surface tension of water by capillary rise
Here is a white paper on General Elasticity of the Material Surface Tension of Water by Capillary Rise:
Introduction:
Capillary rise is a phenomenon where a liquid rises in a narrow tube or channel, driven by the surface tension of the liquid and the properties of the surrounding material. The height to which the liquid rises is determined by the material’s surface properties, including surface tension, wettability, and roughness, as well as the material’s elasticity. The elasticity of the material refers to its ability to deform under stress and recover its original shape when the stress is removed.
This white paper explores the role of the general elasticity of the material in capillary rise and its impact on the performance of various applications.
General Elasticity of the Material:
The general elasticity of a material refers to its ability to deform under stress and recover its original shape when the stress is removed. It is determined by the arrangement of its constituent molecules and how they interact with each other. In general, materials with higher elasticity have a more ordered structure that allows them to deform and recover their original shape more easily.
The general elasticity of the material indirectly affects the capillary rise if it affects the material’s surface properties. For example, a material with a more ordered and regular surface structure is more likely to have lower surface tension, making it easier for the liquid to rise in the capillary tube. Similarly, a material with a rougher or more irregular surface structure may have higher surface tension and lower wettability, which can impede capillary rise.
Capillary Rise Applications:
Capillary rise is a critical phenomenon in various applications, including microfluidic devices, paper chromatography, soil moisture measurement, and oil recovery. In each of these applications, the material’s surface properties, including surface tension and wettability, play a critical role in determining the capillary rise height.
In microfluidic devices, capillary rise enables fluid transport and manipulation in small channels and tubes. The height to which a liquid rises in a capillary tube is determined by the surface properties of the material, including surface tension, wettability, and roughness, as well as the material’s elasticity. Selecting the appropriate material with the right balance of elasticity and surface properties is critical for the efficient and reliable performance of microfluidic devices.
In paper chromatography, capillary rise allows for the separation of different compounds in a mixture. The height to which a liquid rises in the paper is determined by the material’s surface properties, including surface tension, wettability, and roughness. The use of materials with the right balance of elasticity and surface properties can improve the separation efficiency and accuracy.
In soil moisture measurement, capillary rise enables the measurement of the moisture content of soil samples. The height to which a liquid rises in a capillary tube is determined by the surface properties of the soil particles, including surface tension, wettability, and roughness. The use of materials with the right balance of elasticity and surface properties can improve the accuracy and reliability of soil moisture measurements.
In oil recovery, capillary rise allows for the extraction of oil from reservoirs. The height to which a liquid rises in a capillary tube is determined by the surface properties of the rock particles, including surface tension, wettability, and roughness. The use of materials with the right balance of elasticity and surface properties can improve the efficiency and yield of oil recovery processes.
Conclusion:
The general elasticity of the material is an important factor in determining the surface properties, including surface tension, wettability, and roughness, which indirectly affects the capillary rise of a liquid in a narrow tube or channel. The choice of the appropriate material with the right balance of elasticity and surface properties is critical for the efficient and reliable performance of various applications, including microfluidic devices, paper chromatography, soil moisture measurement, and oil recovery. Further research and development in this field could lead to the discovery of new materials with optimized surface properties that could enhance the performance and efficiency of capillary rise applications.
