Glycoside formation is a chemical reaction in which a sugar molecule (i.e., a saccharide) reacts with another molecule (usually an alcohol or phenol) to form a glycoside. Glycosides are a type of organic molecule that are widely found in nature, particularly in plants, where they can serve as storage compounds or structural components of cell walls.
The process of glycoside formation involves the condensation of the hydroxyl group of a sugar molecule with the hydroxyl group of another molecule, usually an alcohol or phenol. This reaction is typically catalyzed by an acid catalyst, such as sulfuric acid, or by enzymes called glycosyltransferases.
The resulting glycoside molecule consists of a sugar molecule (the glycone) attached to another molecule (the aglycone) via a glycosidic bond. The type of glycosidic bond formed depends on the position of the hydroxyl group on the sugar molecule that is involved in the reaction.
Glycosides have a wide range of biological activities and are used in medicine, food science, and other industries. For example, many plant glycosides have medicinal properties and are used as drugs to treat various diseases, such as heart disease, cancer, and diabetes. Glycosides are also commonly found in food and beverages, such as tea, coffee, and wine, where they contribute to flavor and aroma.
What is Required Biomolecules Glycoside formation
The biomolecules required for glycoside formation are a sugar molecule (i.e., a saccharide) and another molecule (usually an alcohol or phenol) to which the sugar will attach to form a glycoside. The sugar molecule is typically a monosaccharide or a disaccharide, such as glucose, fructose, or sucrose. The alcohol or phenol molecule is typically a primary or secondary alcohol or a phenol with a hydroxyl group.
The formation of a glycoside involves the condensation of the hydroxyl group of the sugar molecule with the hydroxyl group of the alcohol or phenol molecule. This reaction typically requires the presence of an acid catalyst, such as sulfuric acid, or an enzymatic catalyst, such as glycosyltransferase, to facilitate the reaction.
In addition to the biomolecules required for glycoside formation, the reaction may also require other chemical reagents or solvents, depending on the specific conditions of the reaction. For example, a common method for synthesizing glycosides involves the use of a protecting group on the hydroxyl group of the sugar molecule to prevent unwanted reactions during the glycoside formation. This protecting group is typically removed in a subsequent step to yield the final glycoside product.
When is Required Biomolecules Glycoside formation
Glycoside formation can occur in a variety of biological and chemical contexts. In nature, glycoside formation is a common process in plants and microorganisms, where it plays a role in storage and structural functions.
In chemical synthesis, glycoside formation is commonly used to produce glycosides for various applications in medicine, food science, and other industries. For example, many plant glycosides have medicinal properties and are used as drugs to treat various diseases, such as heart disease, cancer, and diabetes. Glycosides are also commonly found in food and beverages, such as tea, coffee, and wine, where they contribute to flavor and aroma.
The conditions required for glycoside formation depend on the specific reactants and the desired product. Generally, glycoside formation requires the presence of an acid catalyst or an enzymatic catalyst to facilitate the reaction. The reaction is typically carried out under mild conditions, such as room temperature or slightly elevated temperatures, and in a suitable solvent to promote the reaction.
Where is Required Biomolecules Glycoside formation
Glycoside formation can occur in various locations, both in nature and in chemical synthesis. In nature, glycoside formation takes place primarily in plants and microorganisms. For example, plants use glycoside formation to produce compounds that serve as storage compounds or structural components of cell walls.
In chemical synthesis, glycoside formation can take place in a laboratory setting, usually in a fume hood or other suitable workspace. The reactants are typically mixed together in a suitable solvent and the reaction is carried out under controlled conditions, such as temperature and pressure, to achieve the desired product.
Glycoside formation can also occur in vivo in living organisms. For example, enzymes called glycosyltransferases catalyze the formation of glycosidic bonds between sugars and other biomolecules in cells. This process plays an essential role in various biological processes, such as cell signaling, metabolism, and immune response.
How is Required Biomolecules Glycoside formation
The process of glycoside formation involves the condensation of a sugar molecule with another molecule (usually an alcohol or phenol) to form a glycoside. The reaction is typically catalyzed by an acid catalyst, such as sulfuric acid, or an enzymatic catalyst, such as glycosyltransferase.
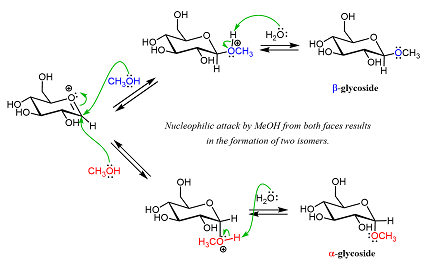
The mechanism of glycoside formation varies depending on the specific reactants and catalysts involved. However, in general, the process involves the nucleophilic attack of the hydroxyl group of the alcohol or phenol on the anomeric carbon of the sugar molecule. This results in the formation of a glycosidic bond between the two molecules, with the sugar molecule acting as the glycone and the alcohol or phenol molecule acting as the aglycone.
In chemical synthesis, glycoside formation can be carried out using various methods, including the use of acid catalysts, enzymatic catalysts, or other chemical reagents to facilitate the reaction. The reaction conditions, such as temperature, pressure, and solvent, can also be varied to optimize the yield and purity of the glycoside product.
In living organisms, glycoside formation is typically catalyzed by enzymes called glycosyltransferases, which transfer sugar residues from activated sugar donors to acceptor molecules. These enzymes play a crucial role in various biological processes, such as cell signaling, metabolism, and immune response.
Structures of Biomolecules Glycoside formation
Glycosides are biomolecules formed by the reaction of a sugar molecule (the glycone) with another molecule (the aglycone or genin) through a glycosidic bond. The glycosidic bond is formed between the anomeric carbon of the sugar and the hydroxyl group of the aglycone.
There are several types of glycosides, including simple glycosides, which contain only one sugar molecule, and complex glycosides, which contain multiple sugar molecules. Examples of simple glycosides include salicin, which is found in willow bark, and digitalin, which is found in foxglove. Examples of complex glycosides include heparin, which is found in the liver and lungs, and glycosaminoglycans, which are found in connective tissue.
The formation of glycosides can be catalyzed by enzymes, such as glycosyltransferases, or can occur spontaneously under certain conditions. The reaction usually involves the displacement of a leaving group, such as a water molecule, from the anomeric carbon of the sugar, resulting in the formation of a covalent bond with the hydroxyl group of the aglycone.
Glycosides play important roles in biological processes, including energy storage, signaling, and structural support. They are also commonly used in medicine, as many drugs are glycosides or contain glycosidic bonds.
Case Study on Biomolecules Glycoside formation
One example of the importance of glycoside formation in biology is the production of the glycoside amygdalin in the seeds of apricots, almonds, and other stone fruits. Amygdalin is a glycoside composed of a cyanogenic glycoside (the aglycone) and a sugar molecule (the glycone).
When the seed is crushed or chewed, the enzyme beta-glucosidase breaks the glycosidic bond, releasing hydrogen cyanide gas. This is a natural defense mechanism for the plant, as hydrogen cyanide is toxic to many animals, including insects and mammals.
In humans, the breakdown of amygdalin can be dangerous if consumed in large quantities, leading to symptoms such as headache, dizziness, and even death in extreme cases. However, small amounts of amygdalin are commonly used in traditional medicine for the treatment of cancer, although its effectiveness is not supported by scientific evidence.
The formation of glycosides is also important in the biosynthesis of complex carbohydrates, such as starch and glycogen. These polymers are composed of glucose molecules linked together by glycosidic bonds, with alpha glycosidic bonds linking glucose molecules in starch and beta glycosidic bonds linking glucose molecules in glycogen.
The formation of glycosidic bonds is catalyzed by enzymes such as glycosyltransferases, which transfer sugar molecules from a nucleotide sugar donor to an acceptor molecule, such as a growing polysaccharide chain. The specificity of glycosyltransferases determines the structure of the resulting carbohydrate, with different enzymes producing different types of glycosidic bonds and linking different sugar molecules together.
In conclusion, glycoside formation is a fundamental process in the biosynthesis of complex carbohydrates and the production of natural defense compounds in plants. The study of glycoside formation and the enzymes involved in this process has important implications for fields such as medicine and biotechnology.
White paper on Biomolecules Glycoside formation
Introduction
Glycoside formation is a biochemical process in which a sugar molecule (the glycone) is linked to another molecule (the aglycone or genin) through a glycosidic bond. The glycosidic bond is formed between the anomeric carbon of the sugar and the hydroxyl group of the aglycone. Glycosides play an important role in biological processes, including energy storage, signaling, and structural support. In this white paper, we will explore the mechanism of glycoside formation, its importance in biology, and its applications in medicine and biotechnology.
Mechanism of glycoside formation
Glycoside formation can be catalyzed by enzymes, such as glycosyltransferases, or can occur spontaneously under certain conditions. The reaction usually involves the displacement of a leaving group, such as a water molecule, from the anomeric carbon of the sugar, resulting in the formation of a covalent bond with the hydroxyl group of the aglycone. The stereochemistry of the anomeric carbon can determine the type of glycoside formed, with alpha glycosidic bonds forming when the anomeric carbon is in the axial position and beta glycosidic bonds forming when the anomeric carbon is in the equatorial position.
Importance of glycoside formation in biology
Glycosides play important roles in biological processes, including energy storage, signaling, and structural support. For example, complex carbohydrates such as starch and glycogen are composed of glucose molecules linked together by glycosidic bonds. Starch is used by plants as a storage form of energy, while glycogen is used by animals as a storage form of glucose.
Glycosides also play a role in signaling, as they can be recognized by specific proteins and used to transmit information between cells. For example, glycosylation of proteins can affect their stability, activity, and interactions with other molecules.
Glycosides can also serve as natural defense compounds in plants, protecting them from predators and pathogens. Cyanogenic glycosides, such as amygdalin in the seeds of apricots and almonds, release hydrogen cyanide gas when broken down, which is toxic to many animals, including insects and mammals.
Applications of glycoside formation in medicine and biotechnology
The formation of glycosides is important in the synthesis of many drugs, as many drugs are glycosides or contain glycosidic bonds. For example, the cardiac glycoside digoxin is used to treat heart failure, while the antiviral drug acyclovir contains a nucleoside glycoside.
The specificity of glycosyltransferases can be exploited in biotechnology to produce specific glycosidic linkages for a variety of applications. For example, glycosylation of proteins can improve their solubility, stability, and bioactivity, making them useful in pharmaceuticals and biotechnology. Glycosylation can also be used to produce vaccines, as glycosylated proteins can induce a stronger immune response than non-glycosylated proteins.
Conclusion
In conclusion, glycoside formation is a fundamental process in biology with important implications for medicine and biotechnology. The formation of glycosidic bonds can be catalyzed by enzymes or can occur spontaneously, resulting in the formation of complex carbohydrates and natural defense compounds in plants. The specificity of glycosyltransferases can be exploited in biotechnology to produce specific glycosidic linkages for a variety of applications, including the synthesis of drugs, vaccines, and glycosylated proteins with improved solubility, stability, and bioactivity.