Chemical and ionic equilibrium are important topics in chemistry and play a significant role in the JEE (Main+Advance) exam. As a repeater, it’s important to have a strong understanding of these concepts and their applications in problem-solving.
Here are some key concepts you should focus on while preparing for JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium:
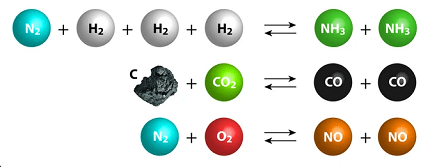
- Law of Mass Action: The law of mass action is an important principle that describes the equilibrium condition of a chemical reaction. It states that the rate of a chemical reaction is proportional to the product of the concentrations of the reactants raised to their stoichiometric coefficients.
- Equilibrium Constant: The equilibrium constant (Kc) is a numerical value that represents the ratio of the concentration of the products to the concentration of the reactants at equilibrium. It provides information about the extent of the reaction and whether the reaction favors the forward or reverse direction.
- Le Chatelier’s Principle: Le Chatelier’s Principle is a fundamental concept in chemical equilibrium that states that when a system at equilibrium is disturbed, it will adjust itself to minimize the effect of the disturbance. This principle can be used to predict the direction of the reaction and the effect of changes in concentration, pressure, and temperature on the equilibrium constant.
- Acid-Base Equilibria: The concepts of acid-base equilibria are crucial in chemistry. You should understand the Bronsted-Lowry acid-base theory, the pH scale, and the equilibrium constant for acid-base reactions.
- Solubility Equilibria: Solubility equilibria describe the equilibrium between a solid and its ions in a solution. The solubility product (Ksp) is the equilibrium constant for this reaction and provides information about the solubility of a compound.
- Common Ion Effect: The common ion effect occurs when an ionic compound is added to a solution containing one of its constituent ions. This effect can shift the equilibrium position of the reaction and should be considered when calculating the equilibrium constant.
- Ionic Equilibria in Aqueous Solutions: You should also have a good understanding of the ionic equilibria that occur in aqueous solutions, such as the hydrolysis of salts and the effect of complex ions on equilibrium.
To prepare for JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium, you should focus on mastering the above concepts and their applications in problem-solving. You can use textbooks, online resources, and practice problems to reinforce your understanding of these topics.
History of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Joint Entrance Examination) is an engineering entrance exam conducted in India for admission into various undergraduate engineering programs. The JEE Main and JEE Advanced are the two stages of the exam.
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium has been a part of the JEE syllabus for many years. However, the syllabus and the emphasis on different topics have changed over time.
The JEE (Main+Advance) exam was first introduced in 2002, replacing the earlier AIEEE (All India Engineering Entrance Examination) and IIT-JEE (Indian Institute of Technology Joint Entrance Examination). The JEE (Main+Advance) exam is conducted by the National Testing Agency (NTA) and is one of the most competitive exams in India.
Chemical and ionic equilibrium have been important topics in the JEE (Main+Advance) exam since its inception. However, the syllabus for the exam has undergone changes over the years, with different topics being emphasized in different years.
For example, in 2019, the JEE (Main+Advance) exam placed a greater emphasis on physical chemistry, with topics such as thermodynamics and electrochemistry being given greater weightage. However, chemical and ionic equilibrium remained important topics and were included in the syllabus.
In recent years, there has been a trend towards asking more application-based questions in the JEE (Main+Advance) exam. This has meant that candidates need to have a strong understanding of the fundamental concepts of chemical and ionic equilibrium and be able to apply them in problem-solving.
Overall, the history of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium has been one of consistent importance and emphasis. Candidates who are preparing for the exam need to have a strong understanding of these topics and be able to apply them effectively in order to succeed.
Importance of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium is a crucial topic for JEE aspirants, as it forms an important part of the exam syllabus. Here are some reasons why this topic is important:
- Weightage in the Exam: Chemical and ionic equilibrium is an important topic in the JEE (Main+Advance) exam and carries a significant weightage. Questions from this topic are often included in the exam and are known to be challenging. Therefore, having a strong understanding of this topic is crucial for achieving a good score.
- Importance in Engineering: Chemical and ionic equilibrium is an important topic in the field of engineering. Understanding the concepts of chemical and ionic equilibrium is important for many applications in engineering, such as the design of chemical processes and the development of materials with specific properties.
- Basis for Further Learning: Chemical and ionic equilibrium is a fundamental concept in chemistry, and a strong foundation in this topic can help students to better understand more advanced topics in chemistry and related fields. For example, understanding the principles of chemical and ionic equilibrium is crucial for topics such as electrochemistry and chemical kinetics.
- Real-World Applications: Chemical and ionic equilibrium has many real-world applications, such as in the design of pharmaceuticals, the development of new materials, and the study of environmental chemistry. Therefore, a good understanding of this topic is not only important for the JEE exam but also for future study and professional development.
In conclusion, JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium is an important topic for JEE aspirants, as it forms an essential part of the exam syllabus and has many practical applications in engineering and other fields. Therefore, students who are preparing for the exam should focus on mastering this topic and its applications in problem-solving.
System of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium system covers various topics related to the principles of chemical and ionic equilibrium. Here are some of the topics that are typically included in the system:
- Acids and Bases: The concept of acids and bases is an important part of chemical equilibrium. Students learn about different types of acids and bases, acid-base equilibria, and pH calculations.
- Equilibrium Constants: Equilibrium constants are used to describe the extent of a chemical reaction. Students learn about the different types of equilibrium constants, such as the acid dissociation constant and the solubility product constant.
- Le Chatelier’s Principle: This principle is used to predict the effect of changes in temperature, pressure, and concentration on chemical equilibrium. Students learn how to apply this principle to solve equilibrium problems.
- Ionic Equilibrium: Students learn about the principles of ionic equilibrium, including ionic product of water, common ion effect, and salt hydrolysis.
- Redox Reactions: Redox reactions involve the transfer of electrons between reactants. Students learn about the principles of redox reactions, including oxidation states, balancing redox reactions, and electrochemistry.
- Thermodynamics: Thermodynamics deals with the relationships between energy, heat, and work. Students learn about the principles of thermodynamics, including the first and second laws of thermodynamics, entropy, and Gibbs free energy.
Overall, the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium system is designed to provide students with a strong foundation in the principles of chemical and ionic equilibrium. By mastering these topics, students are better equipped to solve complex problems in chemistry and related fields.
Conclusion of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
In conclusion, JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium is a critical component of the JEE exam syllabus and an essential topic for aspiring engineers and scientists. The system covers a range of topics related to chemical and ionic equilibrium, including acids and bases, equilibrium constants, Le Chatelier’s principle, ionic equilibrium, redox reactions, and thermodynamics. By mastering these concepts, students are better equipped to understand the principles of chemistry and their real-world applications. Furthermore, a strong understanding of chemical and ionic equilibrium is essential for solving complex problems in chemistry and related fields. Therefore, students who are preparing for the JEE (Main+Advance) exam should focus on mastering this topic and its applications in problem-solving.
Overview of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium system is designed to provide students with a comprehensive understanding of the principles of chemical and ionic equilibrium. The system covers a range of topics, including:
- Acids and Bases: This topic covers the principles of acids and bases, acid-base equilibria, and pH calculations.
- Equilibrium Constants: This topic covers the different types of equilibrium constants, including the acid dissociation constant and the solubility product constant.
- Le Chatelier’s Principle: This topic covers the application of Le Chatelier’s principle to predict the effect of changes in temperature, pressure, and concentration on chemical equilibrium.
- Ionic Equilibrium: This topic covers the principles of ionic equilibrium, including the ionic product of water, the common ion effect, and salt hydrolysis.
- Redox Reactions: This topic covers the principles of redox reactions, including oxidation states, balancing redox reactions, and electrochemistry.
- Thermodynamics: This topic covers the principles of thermodynamics, including the first and second laws of thermodynamics, entropy, and Gibbs free energy.
Overall, the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium system provides students with a strong foundation in the principles of chemical and ionic equilibrium. By mastering these concepts, students are better equipped to understand the real-world applications of chemistry and solve complex problems in chemistry and related fields.
Types of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium can be categorized into different types based on the content and focus of the course. Here are some of the common types:
- Basic course: This type of course covers the fundamental concepts of chemical and ionic equilibrium, including acids and bases, equilibrium constants, and Le Chatelier’s principle.
- Advanced course: This type of course builds upon the basic course and covers more advanced topics such as ionic equilibrium, redox reactions, and thermodynamics.
- Problem-solving course: This type of course focuses on solving complex problems related to chemical and ionic equilibrium. Students are taught various techniques and strategies for approaching and solving problems.
- Exam-focused course: This type of course is designed specifically to help students prepare for the JEE (Main+Advance) exam. The course focuses on the topics and types of questions that are typically covered in the exam.
- Comprehensive course: This type of course covers all the topics related to chemical and ionic equilibrium in detail. It is designed to provide students with a thorough understanding of the subject and prepare them for advanced studies in chemistry.
Overall, the type of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium that a student chooses depends on their individual needs and goals. Some students may benefit more from a problem-solving course, while others may need a comprehensive course to build a strong foundation in the subject.
Structures of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium can be structured in various ways, depending on the course content and the teaching approach. Here are some common structures of the course:
- Lecture-based structure: This structure is the traditional approach to teaching and involves the instructor delivering lectures to the students. The lectures cover the various topics related to chemical and ionic equilibrium, and the students take notes and ask questions as needed.
- Problem-based structure: This structure is focused on problem-solving, and the course materials include a range of problems and exercises related to chemical and ionic equilibrium. The instructor guides the students through the problems, explaining the concepts and techniques used to solve them.
- Discussion-based structure: This structure involves discussions between the instructor and the students, with the aim of fostering critical thinking and analysis. The instructor poses questions related to chemical and ionic equilibrium, and the students engage in discussion, debate, and analysis.
- Laboratory-based structure: This structure involves practical experiments in the laboratory to demonstrate the principles of chemical and ionic equilibrium. The students conduct experiments and analyze the results, under the guidance of the instructor.
- Online-based structure: This structure involves the use of online resources, such as videos, interactive tutorials, and online quizzes, to teach the principles of chemical and ionic equilibrium. Students can learn at their own pace and access the materials from anywhere with an internet connection.
Overall, the structure of the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium depends on the needs of the students and the teaching approach adopted by the instructor. Some structures may be more effective for certain students, depending on their learning style and preferences.
Application of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium has a wide range of applications in various fields. Here are some of the common applications:
- Chemical Engineering: The principles of chemical and ionic equilibrium are used in chemical engineering to design and optimize chemical processes. The understanding of equilibrium constants, acid-base equilibria, and thermodynamics is essential for designing chemical reactors and processes.
- Environmental Science: Chemical and ionic equilibrium principles are used in environmental science to study and understand the behavior of pollutants in the environment. Understanding the concepts of pH, acid-base equilibria, and solubility product constant is critical for studying the behavior of pollutants in aquatic systems.
- Biotechnology: The principles of chemical and ionic equilibrium are used in biotechnology for the production of biomolecules such as proteins and enzymes. The understanding of acid-base equilibria, thermodynamics, and redox reactions is essential for optimizing the production of these biomolecules.
- Pharmacy: The principles of chemical and ionic equilibrium are used in pharmacy for the design and development of drug formulations. The understanding of solubility product constant, pH, and acid-base equilibria is essential for designing and optimizing drug formulations.
- Material Science: The principles of chemical and ionic equilibrium are used in material science for the design and development of new materials. The understanding of thermodynamics, solubility product constant, and acid-base equilibria is essential for designing and optimizing materials with desired properties.
Overall, the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium has a wide range of applications in various fields, and mastering the principles of this course is essential for success in these fields.
Nomenclature of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium involves the study of various topics related to chemical and ionic equilibria, and the nomenclature of these topics depends on the specific concepts being studied. Here are some common terms and nomenclatures used in the course:
- Equilibrium constant (K): This term refers to the ratio of the product of the concentrations of the products to the product of the concentrations of the reactants, each raised to the power of its stoichiometric coefficient. The equilibrium constant is a measure of the position of the equilibrium, and it varies with temperature.
- Acid-base equilibria: This term refers to the equilibria between acids and bases, and it involves the transfer of protons between the acid and base. The nomenclature of acid-base equilibria involves terms such as conjugate acid-base pairs, acid dissociation constants (Ka), and base dissociation constants (Kb).
- Redox reactions: This term refers to the reactions involving the transfer of electrons between the reactants. The nomenclature of redox reactions involves terms such as oxidation state, half-reactions, and reduction potentials.
- Solubility equilibria: This term refers to the equilibria between the dissolved and undissolved phases of a solute in a solvent. The nomenclature of solubility equilibria involves terms such as solubility product constant (Ksp), ion product (Q), and the common ion effect.
- Thermodynamics: This term refers to the study of energy transformations in chemical reactions. The nomenclature of thermodynamics involves terms such as enthalpy (H), entropy (S), and Gibbs free energy (G).
Overall, the nomenclature of the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium involves various terms and concepts related to chemical and ionic equilibria, and mastering the nomenclature is essential for understanding and applying the principles of the course.
Career Opportunities of JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium
The JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium provides a strong foundation in the principles of chemical and ionic equilibria, which can lead to a variety of career opportunities in several fields. Here are some of the career opportunities available to graduates of this course:
- Chemical Engineering: Chemical engineers apply the principles of chemistry and engineering to design and optimize chemical processes. Graduates of the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium are well-suited for careers in chemical engineering due to their knowledge of equilibrium constants, acid-base equilibria, and thermodynamics.
- Environmental Science: Environmental scientists study the behavior of pollutants in the environment and develop strategies for mitigating their impact on ecosystems. Graduates of the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium are well-suited for careers in environmental science due to their knowledge of solubility product constants, pH, and acid-base equilibria.
- Biotechnology: Biotechnologists apply the principles of biology and engineering to develop new products and processes based on living organisms. Graduates of the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium are well-suited for careers in biotechnology due to their knowledge of acid-base equilibria, thermodynamics, and redox reactions.
- Pharmacy: Pharmacists play a critical role in the design and development of new drugs and drug formulations. Graduates of the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium are well-suited for careers in pharmacy due to their knowledge of solubility product constants, pH, and acid-base equilibria.
- Material Science: Materials scientists design and develop new materials with desired properties for various applications. Graduates of the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium are well-suited for careers in material science due to their knowledge of thermodynamics, solubility product constants, and acid-base equilibria.
Overall, the JEE (Main+Advance) Repeater Course Chemical and Ionic Equilibrium provides a strong foundation in the principles of chemical and ionic equilibria, which can lead to a variety of exciting career opportunities in several fields.
