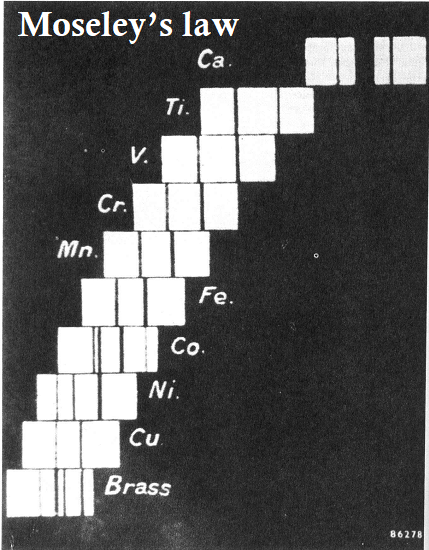
Moseley’s law, also known as Moseley’s law of X-ray spectra, is a scientific law that describes the relationship between the wavelength of an X-ray photon and the atomic number of the element producing the photon. The law was discovered by Henry Moseley, a British physicist, in 1913.
Moseley’s law states that the square root of the frequency of the emitted characteristic X-rays of an element is directly proportional to the atomic number of that element. In mathematical terms, the law can be expressed as:
sqrt(f) = K(Z – σ)
where f is the frequency of the emitted X-ray photon, Z is the atomic number of the element, σ is a constant that depends on the element, and K is a proportionality constant.
Moseley’s law played an important role in the development of the modern understanding of the structure of the atom. By measuring the frequencies of the X-rays emitted by different elements, Moseley was able to show that the atomic number, rather than the atomic weight, is the fundamental property that determines an element’s position in the periodic table. This led to the development of the concept of the atomic number, which is now used to classify elements.
What is Required Moseley’s law
Moseley’s law requires the measurement of the frequency of X-rays emitted by different elements when bombarded with high energy electrons. The law specifically relates to the characteristic X-rays emitted by an element, which are produced when electrons from higher energy levels drop down to lower energy levels in the atom, emitting energy in the form of an X-ray photon.
To measure the X-ray frequencies, a device called a spectrometer is used. The spectrometer separates the X-rays emitted by an element into their component frequencies, allowing the frequencies to be measured and compared to the atomic number of the element.
In order to apply Moseley’s law, a precise measurement of the frequency of X-rays emitted by a particular element is necessary. This requires high-quality experimental equipment and careful data analysis.
Overall, Moseley’s law provides a powerful tool for understanding the properties of atoms and the relationships between elements in the periodic table. It has played a crucial role in the development of our modern understanding of atomic structure and the classification of elements.
When is Required Moseley’s law
Moseley’s law is required in the field of atomic physics and spectroscopy, particularly in the study of X-ray spectra. It is used to determine the atomic number of an element by measuring the frequencies of the X-rays it emits.
Moseley’s law is particularly useful for identifying unknown elements, as each element has a unique set of characteristic X-ray frequencies that can be used to identify it. This can be important in fields such as material science, where the identification of unknown materials is often necessary.
Moseley’s law is also used in X-ray crystallography, which is a technique used to study the structure of crystals. By measuring the X-ray diffraction pattern produced by a crystal, it is possible to determine the arrangement of atoms within the crystal. Moseley’s law can be used to identify the elements present in the crystal, which can provide important information about its structure and properties.
Overall, Moseley’s law is an important tool for understanding the properties of atoms and the behavior of X-rays, and it is used in a variety of fields, including physics, chemistry, material science, and biology.
Where is Required Moseley’s law
Moseley’s law is required in various fields of science where the study of atomic structure and properties is important. Here are some specific examples of where Moseley’s law is used:
- X-ray spectroscopy: Moseley’s law is used to identify and classify elements based on the frequencies of X-rays they emit when bombarded with high-energy electrons. This technique is used in fields such as material science, chemistry, and physics.
- X-ray crystallography: Moseley’s law is used to identify the elements present in crystals, which is important in determining the crystal structure and properties. X-ray crystallography is used in many areas of science, including biology, chemistry, and material science.
- Nuclear physics: Moseley’s law is used in nuclear physics to understand the behavior of atomic nuclei and their interactions with high-energy particles.
- Astrophysics: Moseley’s law is used in astrophysics to study the properties of stars and galaxies. X-rays emitted by these objects can be analyzed using Moseley’s law to determine the elements present and their properties.
Overall, Moseley’s law is an important tool for understanding the properties of atoms and their behavior in a variety of scientific fields.
How is Required Moseley’s law
Moseley’s law is applied through a process called X-ray spectroscopy, which involves bombarding a sample of an element with high-energy electrons to produce characteristic X-rays. The X-rays emitted by the sample are then analyzed using a device called a spectrometer, which separates the X-rays into their component frequencies.
Moseley’s law relates the frequency of the emitted X-rays to the atomic number of the element, allowing the atomic number to be determined from the X-ray frequencies. The law can be expressed mathematically as:
sqrt(f) = K(Z – σ)
where f is the frequency of the emitted X-ray photon, Z is the atomic number of the element, σ is a constant that depends on the element, and K is a proportionality constant.
To apply Moseley’s law, the frequency of the X-rays emitted by a sample of the element must be measured with high precision using a spectrometer. The X-ray frequencies are then compared to a database of known frequencies to determine the atomic number of the element.
In X-ray crystallography, Moseley’s law is used to identify the elements present in a crystal. By measuring the diffraction pattern produced by X-rays passing through the crystal, the positions of the atoms in the crystal can be determined. Moseley’s law can then be used to identify the elements present in the crystal based on the frequencies of the X-rays produced.
Overall, Moseley’s law is applied through the precise measurement of X-ray frequencies and the comparison of these frequencies to known values to determine the atomic number and elemental composition of a sample.
Nomenclature of Moseley’s law
Moseley’s law is named after the English physicist Henry Moseley, who discovered the relationship between the frequency of X-rays emitted by an element and its atomic number in 1913. The law is also sometimes referred to as Moseley’s formula or Moseley’s empirical law.
The law can be expressed mathematically as:
sqrt(f) = K(Z – σ)
where f is the frequency of the emitted X-ray photon, Z is the atomic number of the element, σ is a constant that depends on the element, and K is a proportionality constant.
The square root of the frequency, rather than the frequency itself, is used in the equation to make the relationship between the frequency and the atomic number linear.
The constant σ in the equation is called the screening constant, which takes into account the effect of the electrons in the inner shells of the atom on the frequency of the emitted X-rays. The value of σ varies between different elements and can be experimentally determined.
The proportionality constant K relates the frequency of the emitted X-rays to the atomic number of the element and is also experimentally determined.
Overall, the nomenclature of Moseley’s law reflects its origin and the key parameters that are involved in the relationship between the frequency of emitted X-rays and the atomic number of an element.
Case Study on Moseley’s law
One example of the application of Moseley’s law is in the identification of the element technetium (Tc). Technetium is a radioactive element that was first discovered in 1937 by a team of scientists led by Carlo Perrier and Emilio Segrè. Because of its radioactivity and short half-life, technetium is not found in nature, and its properties were not well understood until its discovery.
After its discovery, scientists were able to study the X-ray spectrum of technetium and found that it emitted a set of characteristic X-rays that did not correspond to any known element. This led to the development of Moseley’s law, which allowed the atomic number of technetium to be determined from the frequency of its X-rays.
Using Moseley’s law, scientists were able to determine that technetium had an atomic number of 43, which made it the first element discovered through spectroscopic analysis rather than chemical methods. Technetium has since been used in a variety of applications, including medical imaging and nuclear power generation.
Another example of the application of Moseley’s law is in the study of the crystal structure of proteins. Proteins are complex molecules made up of amino acids, and their function is dependent on their three-dimensional structure. X-ray crystallography is a technique used to determine the structure of proteins by analyzing the diffraction pattern produced by X-rays passing through a crystal of the protein.
Moseley’s law is used in X-ray crystallography to identify the elements present in the crystal, which is important in determining the structure of the protein. By analyzing the diffraction pattern produced by the X-rays passing through the crystal, the positions of the atoms in the crystal can be determined. Moseley’s law can then be used to identify the elements present in the crystal based on the frequencies of the X-rays produced.
Overall, the application of Moseley’s law has had a significant impact on the study of atomic structure and properties, particularly in the fields of spectroscopy, crystallography, and nuclear physics. By allowing the atomic number of an element to be determined from the frequency of its emitted X-rays, Moseley’s law has enabled scientists to identify new elements and gain a deeper understanding of the properties of atoms and molecules.
White paper on Moseley’s law
Introduction:
Moseley’s law is a fundamental principle in the field of atomic physics that relates the frequency of the emitted X-rays from an element to its atomic number. The law was discovered by the English physicist Henry Moseley in 1913 and provided a crucial tool for the study of atomic structure and properties. This white paper will provide an in-depth exploration of Moseley’s law, its history, applications, and relevance in modern science.
Discovery and development of Moseley’s law:
Moseley’s law was discovered by Henry Moseley in 1913, while he was studying the X-ray spectra of various elements. Moseley observed that the frequencies of the emitted X-rays were proportional to the square of the atomic number of the element rather than its atomic weight. He hypothesized that this relationship was due to the fact that the atomic number of an element corresponds to the number of protons in its nucleus.
Moseley’s law was further developed by other scientists in the following years, including Niels Bohr, who used it to refine his model of the atom. The law also played a crucial role in the discovery of new elements, such as technetium and promethium, and in the development of X-ray crystallography, a technique used to study the structure of molecules.
Applications of Moseley’s law:
Moseley’s law has numerous applications in the fields of atomic physics, spectroscopy, crystallography, and nuclear physics. One important application of Moseley’s law is in the identification of unknown elements, particularly those that are not found in nature. By measuring the frequency of the X-rays emitted by a sample of the element and applying Moseley’s law, scientists can determine the atomic number and therefore the identity of the element.
Moseley’s law is also used in X-ray crystallography, a technique used to determine the structure of molecules. By analyzing the diffraction pattern produced by X-rays passing through a crystal of the molecule, scientists can determine the positions of the atoms in the molecule. Moseley’s law is then used to identify the elements present in the molecule based on the frequencies of the emitted X-rays.
In nuclear physics, Moseley’s law is used to study the properties of atomic nuclei. By bombarding nuclei with high-energy particles, scientists can study the frequencies of the emitted X-rays and apply Moseley’s law to determine the atomic number and other properties of the nucleus.
Relevance in modern science:
Moseley’s law remains a crucial tool in modern science, particularly in the study of atomic structure and properties. The law has enabled the discovery of new elements and provided insights into the behavior of atoms and molecules. It has also played a crucial role in the development of X-ray crystallography, a technique that has revolutionized the study of molecular structure.
Conclusion:
Moseley’s law is a fundamental principle in the field of atomic physics that relates the frequency of the emitted X-rays from an element to its atomic number. The law has numerous applications in the fields of spectroscopy, crystallography, and nuclear physics and has played a crucial role in the discovery of new elements and the study of atomic structure and properties. Despite being over a century old, Moseley’s law remains a crucial tool in modern science and will continue to play an important role in the development of new technologies and the understanding of the natural world.