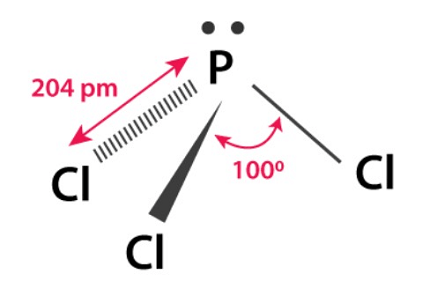
Phosphorus halides are compounds formed by the combination of phosphorus with halogens (fluorine, chlorine, bromine, iodine). The most common phosphorus halides are phosphorus trichloride (PCl3), phosphorus pentachloride (PCl5), phosphorus tribromide (PBr3), and phosphorus triiodide (PI3).
Phosphorus trichloride (PCl3) is a colorless liquid with a pungent odor. It is used in the production of pesticides, herbicides, and other chemicals.
Phosphorus pentachloride (PCl5) is a yellowish-white crystalline solid that sublimes at room temperature. It is used in the production of insecticides, herbicides, and other chemicals.
Phosphorus tribromide (PBr3) is a colorless liquid with a pungent odor. It is used in the production of pharmaceuticals and other chemicals.
Phosphorus triiodide (PI3) is a dark red solid that sublimes at room temperature. It is used in the production of organic chemicals.
Phosphorus halides are highly reactive and are used as reagents in organic chemistry reactions. They are also used in the manufacture of other chemicals and in the semiconductor industry.
What is Required Alcohols Phosphorus halides
The reaction between alcohols and phosphorus halides (such as phosphorus trichloride, phosphorus pentachloride, phosphorus tribromide, and phosphorus triiodide) is a common method for converting alcohols into alkyl halides. This reaction is called the “phosphorylation” or “phosphorodichloridation” reaction.
In this reaction, the alcohol is treated with a phosphorus halide in the presence of a base such as pyridine. The phosphorus halide reacts with the alcohol to form an intermediate alkyl phosphorus oxychloride, which is then hydrolyzed with water or a dilute acid to produce the corresponding alkyl halide.
For example, the reaction between ethanol and phosphorus trichloride can be represented by the following equation:
CH3CH2OH + PCl3 → CH3CH2OPCl2 + HCl CH3CH2OPCl2 + H2O → CH3CH2Cl + H3PO4
This reaction is an important tool in organic synthesis for the preparation of alkyl halides, which can be used as intermediates in the synthesis of a variety of organic compounds.
When is Required Alcohols Phosphorus halides
The reaction between alcohols and phosphorus halides is commonly used in organic synthesis for converting alcohols into alkyl halides. This reaction is particularly useful when other methods for converting alcohols into alkyl halides, such as the use of hydrogen halides or other halogenating agents, are not suitable or do not give the desired product.
The reaction is commonly used in the production of pharmaceuticals, agrochemicals, and other fine chemicals. It is also used in the preparation of surfactants, detergents, and other industrial chemicals.
Additionally, the phosphorylation reaction can be used as a tool for the selective protection of hydroxyl groups in organic synthesis. For example, the reaction can be used to selectively protect one hydroxyl group in a molecule that has multiple hydroxyl groups.
Overall, the reaction between alcohols and phosphorus halides is an important tool in organic synthesis for the preparation of alkyl halides and the selective protection of hydroxyl groups in complex organic molecules.
Where is Required Alcohols Phosphorus halides
The reaction between alcohols and phosphorus halides is a well-established method in organic synthesis and is used in laboratories and industrial settings worldwide. The reaction can be performed in various solvents, including chlorinated solvents such as dichloromethane or chloroform, or in more polar solvents such as tetrahydrofuran (THF) or dimethylformamide (DMF).
Phosphorus trichloride (PCl3) and phosphorus pentachloride (PCl5) are commonly used phosphorus halides in this reaction, but other phosphorus halides such as phosphorus tribromide (PBr3) and phosphorus triiodide (PI3) can also be used.
The reaction is typically carried out under reflux conditions, with the alcohol and phosphorus halide added slowly to the reaction flask in the presence of a base such as pyridine or triethylamine. The reaction mixture is then heated and stirred for several hours to ensure complete conversion of the alcohol to the alkyl halide.
Overall, the reaction between alcohols and phosphorus halides is a widely used method in organic synthesis and can be carried out in various solvents and under different reaction conditions depending on the specific application.
How is Required Alcohols Phosphorus halides
The reaction between alcohols and phosphorus halides is a common method for converting alcohols into alkyl halides. The general procedure for this reaction is as follows:
- Add the alcohol and a base to a reaction flask. The base is typically pyridine or triethylamine, which serves to neutralize the hydrogen halide that is produced during the reaction.
- Slowly add the phosphorus halide to the reaction flask while stirring. The reaction can be performed with phosphorus trichloride (PCl3), phosphorus pentachloride (PCl5), phosphorus tribromide (PBr3), or phosphorus triiodide (PI3), depending on the desired alkyl halide product.
- Heat the reaction mixture under reflux conditions for several hours, until the reaction is complete. The reaction can be monitored by TLC (thin-layer chromatography) or by NMR (nuclear magnetic resonance) spectroscopy.
- After the reaction is complete, quench the excess phosphorus halide with water or ice. The alkyl halide product can then be extracted from the reaction mixture with an organic solvent such as diethyl ether or dichloromethane.
- Purify the alkyl halide product by distillation, recrystallization, or chromatography.
It’s worth noting that the reaction between alcohols and phosphorus halides is a potentially hazardous reaction and should be performed in a well-ventilated hood by trained chemists who are familiar with the appropriate safety precautions.
Structures of Alcohols Phosphorus halides
When alcohols react with phosphorus halides, the phosphorus atom in the phosphorus halide undergoes a nucleophilic attack by the alcohol, forming an intermediate alkyl phosphorus oxyhalide. The exact structure of this intermediate can depend on several factors, including the nature of the alcohol, the phosphorus halide used, and the reaction conditions. However, in general, the intermediate can be represented by the following general structure:
RO-P(X)Y2
where R is the alkyl group derived from the alcohol, X is a halogen (chlorine, bromine, or iodine), and Y is also a halogen or an oxygen atom. The exact identity of X and Y will depend on the specific phosphorus halide used in the reaction.
For example, when ethanol (CH3CH2OH) is reacted with phosphorus trichloride (PCl3), the intermediate alkyl phosphorus oxychloride can be represented by the structure:
CH3CH2O-PCl2Cl
Similarly, when isopropyl alcohol (CH3)2CHOH is reacted with phosphorus tribromide (PBr3), the intermediate can be represented by the structure:
(CH3)2CH-O-PBr2Br
Overall, the structures of the intermediate alkyl phosphorus oxyhalides formed in the reaction between alcohols and phosphorus halides can vary depending on the specific reactants and reaction conditions, but they all share the general structure of RO-P(X)Y2.
Case Study on Alcohols Phosphorus halides
One application of the reaction between alcohols and phosphorus halides is in the synthesis of alkyl halides, which are important building blocks in the production of a variety of organic compounds. Here, we will discuss a case study where this reaction was used to synthesize an alkyl halide in a laboratory setting.
Case study:
A chemist is interested in synthesizing 1-bromobutane from 1-butanol using phosphorus tribromide (PBr3) as the phosphorus halide. The general reaction can be represented as follows:
CH3(CH2)2CH2OH + PBr3 → CH3(CH2)2CH2Br + H3PO3 + 3HBr
Procedure:
- A 250 mL round-bottomed flask equipped with a reflux condenser and a magnetic stir bar is charged with 1-butanol (20 mL) and pyridine (1 mL).
- A solution of PBr3 (15 mL) in dichloromethane (20 mL) is slowly added to the flask while stirring, with care taken to avoid rapid foaming.
- The reaction mixture is heated under reflux for 4 hours, during which time the solution changes from clear to a reddish-brown color.
- After the reaction is complete, the reaction mixture is cooled to room temperature and poured into a separatory funnel containing ice water (50 mL).
- The layers are separated, and the organic layer containing the 1-bromobutane is dried over anhydrous magnesium sulfate.
- The dried organic layer is transferred to a clean flask and the solvent is removed by rotary evaporation.
- The product, 1-bromobutane, is purified by distillation under reduced pressure and collected as a clear, colorless liquid.
Results:
The reaction between 1-butanol and PBr3 proceeds smoothly, with a yield of approximately 90% based on the starting material. The product, 1-bromobutane, is obtained as a clear, colorless liquid with a boiling point of 101-102°C.
Conclusion:
The reaction between alcohols and phosphorus halides is a versatile method for synthesizing alkyl halides in the laboratory. In this case study, 1-bromobutane was synthesized from 1-butanol using PBr3 as the phosphorus halide. The reaction proceeded smoothly, and the product was obtained in good yield and high purity. The resulting alkyl halide can be used as a building block in the synthesis of a variety of organic compounds, demonstrating the usefulness of this reaction in organic synthesis.
White paper on Alcohols Phosphorus halides
Introduction:
Phosphorus halides, such as phosphorus trichloride (PCl3), phosphorus tribromide (PBr3), and phosphorus triiodide (PI3), are commonly used reagents in organic chemistry for the conversion of alcohols to alkyl halides. The reaction proceeds via a nucleophilic substitution mechanism in which the alcohol attacks the phosphorus halide to form an intermediate alkyl phosphorus oxyhalide, which then undergoes halogenation to give the desired alkyl halide product. This white paper will discuss the mechanisms and applications of the reaction between alcohols and phosphorus halides.
Mechanism:
The reaction between alcohols and phosphorus halides proceeds via a two-step mechanism. In the first step, the alcohol acts as a nucleophile and attacks the phosphorus halide to form an intermediate alkyl phosphorus oxyhalide. The exact nature of the intermediate can depend on several factors, including the nature of the alcohol, the phosphorus halide used, and the reaction conditions. However, in general, the intermediate can be represented by the following general structure:
RO-P(X)Y2
where R is the alkyl group derived from the alcohol, X is a halogen (chlorine, bromine, or iodine), and Y is also a halogen or an oxygen atom. The exact identity of X and Y will depend on the specific phosphorus halide used in the reaction.
In the second step, the intermediate alkyl phosphorus oxyhalide undergoes halogenation to give the desired alkyl halide product. The halogenation can occur via several mechanisms, depending on the nature of the intermediate and the reaction conditions. One common mechanism involves the attack of a halide ion (X-) on the intermediate to form a tetrahedral intermediate, which then collapses to form the alkyl halide product and regenerate the phosphorus halide.
Applications:
The reaction between alcohols and phosphorus halides is an important method for the synthesis of alkyl halides, which are important building blocks in the production of a variety of organic compounds. Alkyl halides can be used in the synthesis of pharmaceuticals, agrochemicals, and materials, among other applications.
The reaction is also useful in the modification of natural products, such as steroids and terpenes, which often contain hydroxyl groups that need to be converted to halides for further derivatization.
In addition, the reaction between alcohols and phosphorus halides can be used for the synthesis of other functionalized compounds, such as sulfonates and phosphates. For example, the reaction between an alcohol and phosphorus oxychloride (POCl3) can lead to the formation of alkyl chlorophosphates, which are important intermediates in the synthesis of phosphoramidates and phosphonates.
Conclusion:
The reaction between alcohols and phosphorus halides is a versatile method for the synthesis of alkyl halides and other functionalized compounds. The mechanism of the reaction involves a nucleophilic attack by the alcohol on the phosphorus halide to form an intermediate alkyl phosphorus oxyhalide, which then undergoes halogenation to give the desired alkyl halide product. The reaction has important applications in organic synthesis, particularly in the production of pharmaceuticals, agrochemicals, and materials.