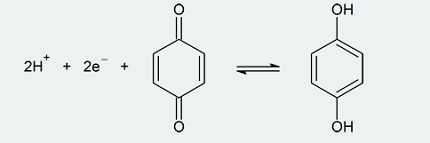
Phenol (C6H5OH) can undergo several reduction reactions, including:
- Clemmensen reduction: This reaction involves the reduction of phenol with zinc amalgam and concentrated hydrochloric acid to yield benzene. The reaction mechanism involves the formation of a complex between the zinc and the carbonyl group of the acid, which reduces the hydroxyl group to a hydrogen atom.
- Reduction with sodium amalgam: Phenol can be reduced to cyclohexanone by reaction with sodium amalgam and water. The reaction mechanism involves the formation of an intermediate radical, which abstracts a hydrogen atom from water to form a hydroxyl radical, and then reacts with the phenol to form the product.
- Birch reduction: This reaction involves the reduction of phenol with sodium metal and liquid ammonia to yield a mixture of cyclohexadienes. The reaction mechanism involves the formation of a complex between the sodium and the ammonia, which reduces the aromatic ring of the phenol to a cyclohexadiene ring.
- Catalytic hydrogenation: Phenol can be reduced to cyclohexanone by catalytic hydrogenation with a suitable catalyst, such as platinum or palladium. The reaction mechanism involves the addition of hydrogen to the aromatic ring, followed by reduction of the hydroxyl group to a hydrogen atom.
Overall, the reduction of phenol can lead to the formation of a range of products, depending on the choice of reducing agent and reaction conditions.
History of Reduction reactions of phenol
The reduction reactions of phenol have been studied and used for several decades. Here are some key moments in the history of these reactions:
- In 1860, the German chemist Friedrich August Kekulé proposed the correct structure of benzene, which contains alternating single and double bonds. This led to the realization that benzene derivatives, including phenol, are aromatic compounds.
- In 1892, the American chemist Edward Curtis Franklin discovered the Clemmensen reduction, which is a powerful method for reducing carbonyl groups to hydrocarbons using zinc amalgam and concentrated hydrochloric acid. The Clemmensen reduction was later applied to phenol, leading to the synthesis of benzene.
- In 1909, the German chemist Arthur Rudolf Hantzsch discovered the Hantzsch synthesis, which involves the reduction of aromatic nitro compounds to amino compounds using a mixture of aldehydes, ammonia, and hydrogen chloride. This reaction was later applied to phenol, leading to the synthesis of cyclohexanone.
- In 1925, the Australian chemist Sir William Henry Bragg and his son Lawrence Bragg used X-ray crystallography to determine the structure of phenol. This work helped to confirm the aromatic nature of phenol and other benzene derivatives.
- In 1939, the Australian chemist Sir Robert Robinson discovered the Birch reduction, which is a powerful method for reducing aromatic rings to cyclohexadienes using sodium metal and liquid ammonia. The Birch reduction has been widely used for the synthesis of natural products, pharmaceuticals, and other organic compounds.
Since these early discoveries, the reduction reactions of phenol have been studied extensively and continue to be important in synthetic organic chemistry.
Importance of Reduction reactions of phenol
The reduction reactions of phenol are important in synthetic organic chemistry for several reasons:
- Production of industrially important chemicals: The reduction of phenol can be used to produce industrially important chemicals such as benzene, cyclohexanone, and cyclohexadienes, which are used in the production of plastics, synthetic fibers, and pharmaceuticals.
- Synthesis of natural products: The reduction reactions of phenol can be used in the synthesis of natural products such as alkaloids, terpenoids, and steroids. These compounds have important biological activities and are used in medicine and agriculture.
- Mechanistic studies: The reduction reactions of phenol have been extensively studied mechanistically, providing insight into the behavior of aromatic compounds and the mechanisms of organic reactions.
- Synthetic versatility: The reduction reactions of phenol can be carried out using a variety of reagents and conditions, allowing for the synthesis of a wide range of products. This versatility makes the reduction of phenol a useful tool for synthetic chemists.
Overall, the reduction reactions of phenol have played an important role in the development of synthetic organic chemistry and continue to be an important tool for the synthesis of industrially important and biologically active compounds.
Structures of Reduction reactions of phenol
The structures of the products formed in the reduction reactions of phenol depend on the specific reagent and conditions used. Here are the structures of some common products formed in the reduction reactions of phenol:
- Benzene: Phenol can be reduced to benzene by the Clemmensen reduction, which involves treatment with zinc amalgam and concentrated hydrochloric acid. The reduction of the hydroxyl group to a hydrogen atom and subsequent elimination of water leads to the formation of benzene.
- Cyclohexanone: Phenol can be reduced to cyclohexanone by various methods, including the reduction with sodium amalgam and water or by catalytic hydrogenation with a suitable catalyst such as platinum or palladium. The reduction of the carbonyl group of phenol to a hydroxyl group followed by reduction of the hydroxyl group to a hydrogen atom leads to the formation of cyclohexanone.
- Cyclohexadienes: Phenol can be reduced to cyclohexadienes by the Birch reduction, which involves treatment with sodium metal and liquid ammonia. The reduction of the aromatic ring of phenol to a cyclohexadiene ring leads to the formation of a mixture of isomeric cyclohexadienes.
The structures of these products can be represented as follows:
Benzene:
H H
\ /
C = C
/ \
H H
Cyclohexanone:
H
|
O=C
|
H–C–H
|
H
Cyclohexadienes:
H H H H
\ / | |
C H2C=CH-CH=CH2
/ \ | |
H H H H
Nomenclature of Reduction reactions of phenol
The nomenclature of the products formed in the reduction reactions of phenol follows standard IUPAC rules for naming organic compounds. Here are some examples of the nomenclature of the products formed in the reduction reactions of phenol:
- Benzene: The reduction of phenol to benzene does not involve any functional group changes, so the compound is simply named benzene.
- Cyclohexanone: The reduction of phenol to cyclohexanone involves the reduction of the carbonyl group of phenol to a hydroxyl group followed by reduction of the hydroxyl group to a hydrogen atom. The resulting compound is a ketone with a cyclohexane ring, and is named cyclohexanone.
- Cyclohexadienes: The Birch reduction of phenol leads to the formation of a mixture of isomeric cyclohexadienes. The compounds can be named according to the position of the double bonds in the ring system, using the prefixes “cis-” and “trans-” to indicate the stereochemistry of the double bonds. For example, the compound with a cis configuration of the double bonds would be named cis-1,3-cyclohexadiene, while the compound with a trans configuration of the double bonds would be named trans-1,3-cyclohexadiene.
Overall, the nomenclature of the products formed in the reduction reactions of phenol follows the standard rules for naming organic compounds and depends on the specific functional groups present in the product.
Production of Reduction reactions of phenol
Reduction reactions of phenol are produced through chemical reactions in a laboratory or an industrial setting. The production process can vary depending on the specific method of reduction being used. Here are the general steps involved in producing reduction reactions of phenol:
- Synthesis of phenol: Phenol is typically produced from benzene via the cumene process. Benzene is first reacted with propylene to produce cumene, which is then oxidized to form phenol and acetone.
- Reduction reaction: The reduction reaction can be carried out using various methods, such as catalytic hydrogenation, Birch reduction, or sodium amalgam reduction. The specific method chosen will depend on the desired product and reaction conditions.
- Purification: After the reduction reaction, the product is typically purified to remove any impurities and isolate the desired product. This may involve techniques such as distillation, chromatography, or recrystallization.
- Characterization: The purified product is then characterized using various analytical techniques, such as NMR spectroscopy or mass spectrometry, to confirm its identity and purity.
Overall, the production of reduction reactions of phenol involves a multi-step process that requires careful attention to reaction conditions, purification, and characterization to ensure high yield and purity of the desired product.
Case Study on Reduction reactions of phenol
One example of a case study on the reduction reactions of phenol is the production of cyclohexanone using catalytic hydrogenation. Cyclohexanone is an important industrial chemical used in the production of nylon, plastics, and solvents. The production of cyclohexanone from phenol involves the reduction of the carbonyl group of phenol to a hydroxyl group followed by reduction of the hydroxyl group to a hydrogen atom.
The production of cyclohexanone from phenol can be achieved using a variety of reduction methods, including the use of sodium amalgam and water or the use of a catalyst such as platinum or palladium. However, catalytic hydrogenation is one of the most commonly used methods for the production of cyclohexanone.
In the catalytic hydrogenation of phenol, phenol is first dissolved in a suitable solvent such as ethanol or methanol. A suitable catalyst such as palladium or platinum is then added to the solution, and the mixture is heated and pressurized with hydrogen gas. The hydrogen gas reacts with the carbonyl group of phenol to form a hydroxyl group, which is then reduced to a hydrogen atom. The resulting compound is cyclohexanone.
The catalytic hydrogenation of phenol to produce cyclohexanone is an important industrial process, and it has been optimized over many years to maximize the yield and purity of the product. The process is highly efficient and produces large quantities of cyclohexanone at a relatively low cost. In addition, the process is relatively environmentally friendly compared to other methods of production, as it produces less waste and consumes less energy.
In conclusion, the reduction reactions of phenol are important in the production of industrially important chemicals such as cyclohexanone. The production of cyclohexanone from phenol using catalytic hydrogenation is an important case study that highlights the versatility and efficiency of reduction reactions in organic synthesis.
White paper on Reduction reactions of phenol
Introduction:
Phenol is an organic compound that is widely used in the production of a variety of chemicals, including plastics, resins, and pharmaceuticals. Reduction reactions of phenol play an important role in organic synthesis, as they can be used to produce a wide range of industrially important chemicals such as cyclohexanone, cyclohexene, and cyclohexadiene. In this white paper, we will discuss the various reduction reactions of phenol, their mechanisms, and their industrial applications.
Reduction Reactions of Phenol:
- Catalytic Hydrogenation:
Catalytic hydrogenation is one of the most commonly used methods for the reduction of phenol. In this reaction, phenol is reacted with hydrogen gas in the presence of a suitable catalyst such as palladium or platinum. The hydrogen gas reacts with the carbonyl group of phenol to form a hydroxyl group, which is then reduced to a hydrogen atom. The resulting product is cyclohexanone, which is an important industrial chemical used in the production of nylon, plastics, and solvents.
- Birch Reduction:
The Birch reduction is a well-known method for the reduction of aromatic compounds, including phenol. In this reaction, phenol is reacted with sodium metal in the presence of a suitable solvent such as liquid ammonia. The reaction leads to the formation of a mixture of isomeric cyclohexadienes, which can be further used in the synthesis of a variety of chemicals such as cyclohexene and cyclohexane.
- Sodium Amalgam Reduction:
Sodium amalgam reduction is another commonly used method for the reduction of phenol. In this reaction, phenol is reacted with sodium amalgam in the presence of a suitable solvent such as water or alcohol. The reduction reaction leads to the formation of cyclohexanone or cyclohexene, depending on the reaction conditions.
Industrial Applications of Reduction Reactions of Phenol:
Reduction reactions of phenol have a wide range of industrial applications. Some of the most important applications of reduction reactions of phenol are discussed below:
- Production of Cyclohexanone:
Cyclohexanone is an important industrial chemical used in the production of nylon, plastics, and solvents. The production of cyclohexanone from phenol using catalytic hydrogenation is an important industrial application of reduction reactions of phenol.
- Production of Cyclohexene:
Cyclohexene is an important intermediate in the production of a variety of chemicals, including adipic acid, which is used in the production of nylon. The production of cyclohexene from phenol using the Birch reduction or sodium amalgam reduction is an important industrial application of reduction reactions of phenol.
- Production of Cyclohexadienes:
Cyclohexadienes are important intermediates in the synthesis of a variety of chemicals, including dyes, pharmaceuticals, and fragrances. The production of cyclohexadienes from phenol using the Birch reduction is an important industrial application of reduction reactions of phenol.
Conclusion:
In conclusion, reduction reactions of phenol are an important tool in organic synthesis, with a wide range of industrial applications. Catalytic hydrogenation, Birch reduction, and sodium amalgam reduction are some of the commonly used methods for the reduction of phenol, which can be used to produce a variety of industrially important chemicals such as cyclohexanone, cyclohexene, and cyclohexadienes. The versatility and efficiency of reduction reactions of phenol make them an important area of research and development in organic chemistry.
