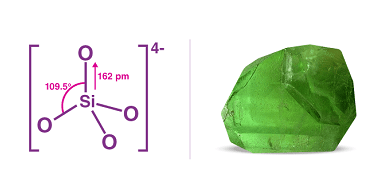
Group 15 Allotropes of phosphorous
Phosphorus is a chemical element with the symbol P and atomic number 15. It has several allotropes, which are different forms of the same element that exist in the same physical state. The most common allotropes of phosphorus are: Each of these allotropes has different physical and chemical properties, making them useful for different applications…