Group 13 Preparation
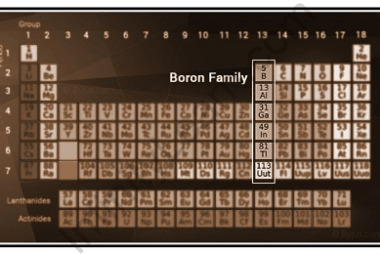
Group 13 of the periodic table consists of Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), and Thallium (Tl). These elements all have three valence electrons in their outermost shell and are referred to as the “p-block” elements. The group 13 elements can be prepared through various methods, depending on the specific element: Overall, the…