JEE (Main+Advance) e-Intermediate Course s-Block Elements
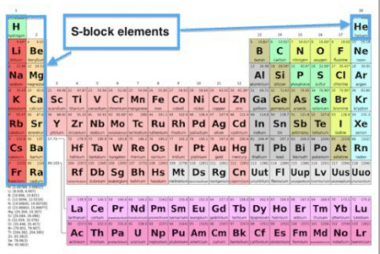
S-block elements are the elements in group 1 and group 2 of the periodic table. They are also known as the alkali metals (group 1) and alkaline earth metals (group 2). These elements have unique chemical and physical properties, such as low ionization energies, high reactivity, and low electronegativities. In the context of JEE (Main+Advanced),…