Wien’s displacement law
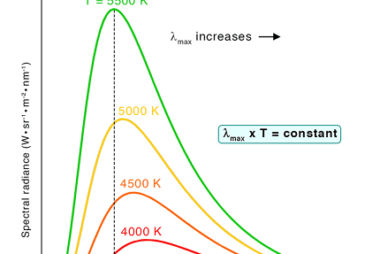
Wien’s displacement law, named after Wilhelm Wien, states that the peak wavelength of radiation emitted by a black body is inversely proportional to its temperature. Mathematically, Wien’s displacement law can be expressed as λ_max = b/T, where λ_max is the peak wavelength of the radiation emitted by the black body, T is its temperature in…