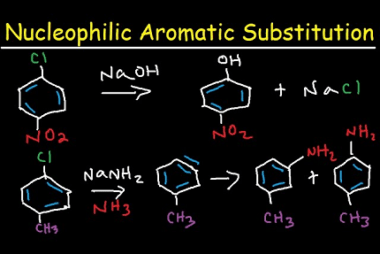
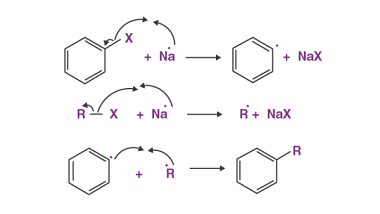
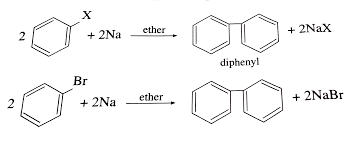
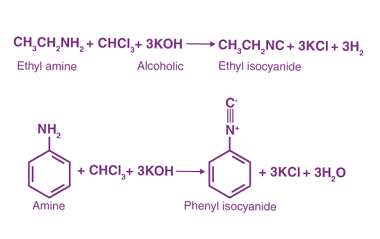
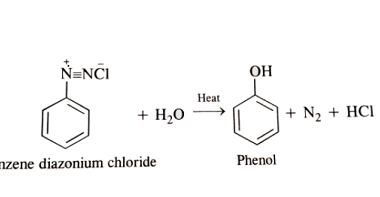
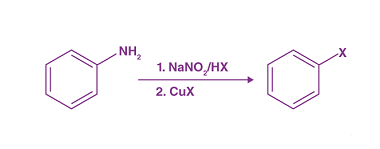
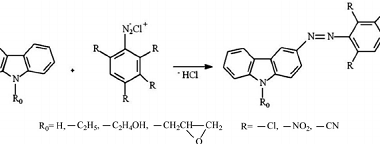
Substituted haloarenes
Substituted haloarenes are a class of organic compounds that contain one or more halogen atoms (such as chlorine, bromine, or iodine) attached to an aromatic ring. These compounds are commonly used as starting materials for the synthesis of a variety of organic compounds, including pharmaceuticals, agrochemicals, and materials. The properties of substituted haloarenes can vary…