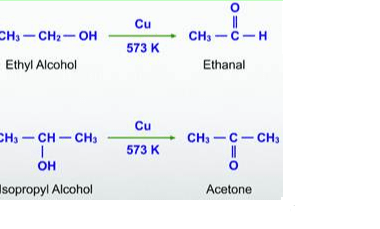
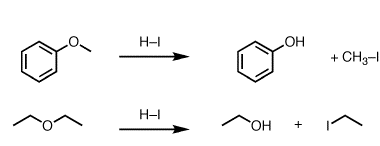
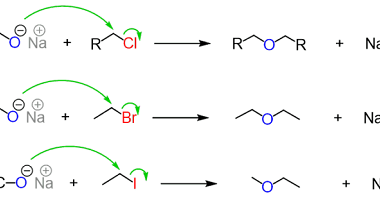
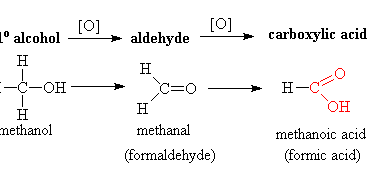
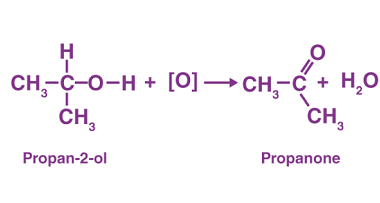
Ketones from acid chlorides
Ketones can be synthesized from acid chlorides through a reaction called Friedel-Crafts acylation. This reaction involves the reaction of an acid chloride with a Lewis acid catalyst, such as aluminum chloride (AlCl3), to form an acylium ion intermediate. The acylium ion then undergoes a nucleophilic attack by an arene, which leads to the formation of…