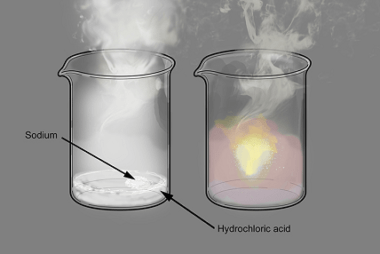
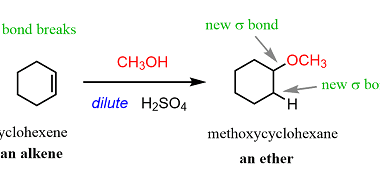
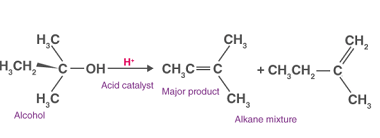
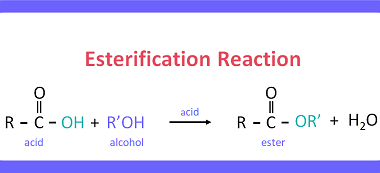
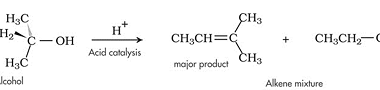
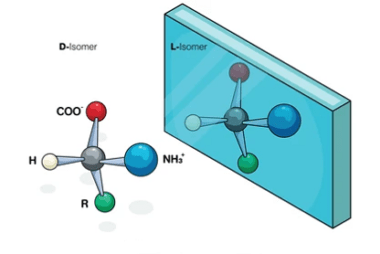
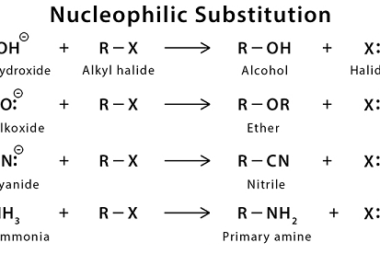
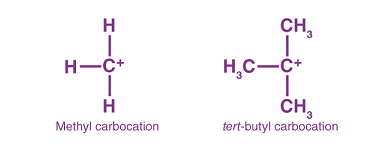Reactions with: Sodium
Sodium is a highly reactive alkali metal with the symbol Na and atomic number 11. It has a silvery-white appearance and is soft enough to be cut with a knife. Sodium reacts vigorously with water to produce sodium hydroxide and hydrogen gas. It also reacts with oxygen to form sodium oxide and with halogens to…