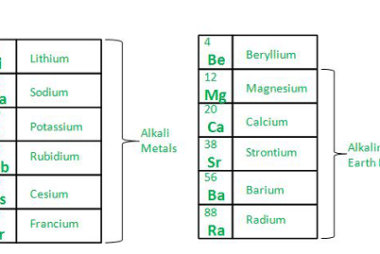
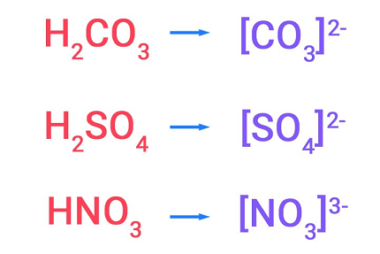
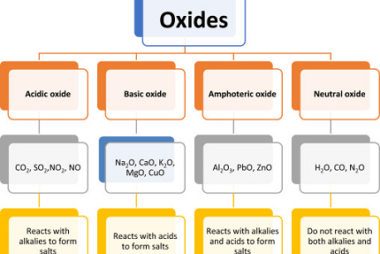
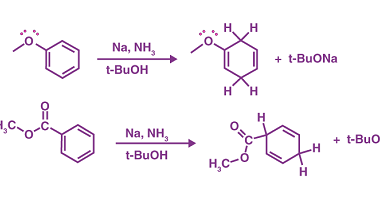
s-Block Elements Preparation
Great! Here are some tips for preparing for s-block elements: By following these tips and putting in consistent effort, you can prepare effectively for s-block elements and perform well on exams or assignments related to this topic. Good luck! What is Required s-Block Elements Preparation To prepare well for s-block elements, you will need to…