Calorimetry
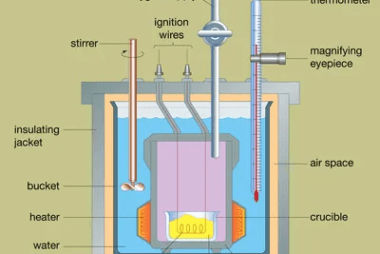
Calorimetry is the scientific measurement of heat transfer in a physical or chemical process. It involves the determination of the amount of heat absorbed or released by a substance or system during a physical or chemical change. Calorimetry is an important tool in various fields of science, including chemistry, physics, and engineering. The basic principle…