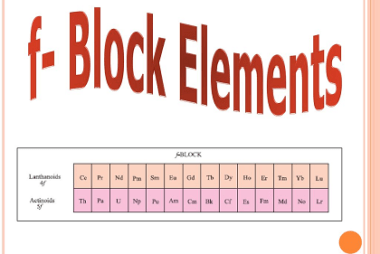
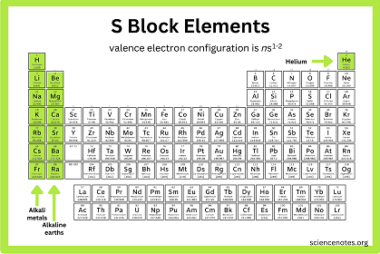
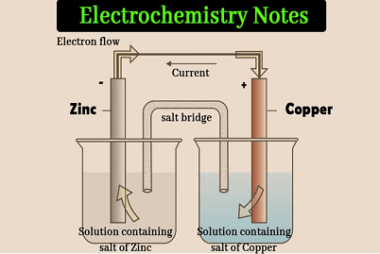
JEE (Main+Advance) Intermediate Course Ethers
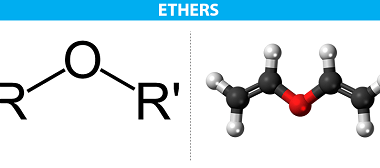
Ethers are a class of organic compounds that contain an oxygen atom bonded to two alkyl or aryl groups. They have the general formula R-O-R’, where R and R’ are alkyl or aryl groups. Ethers are important in organic chemistry because they are widely used as solvents and as intermediates in the synthesis of other…