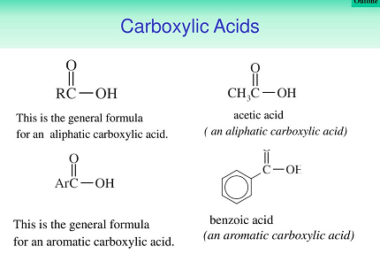
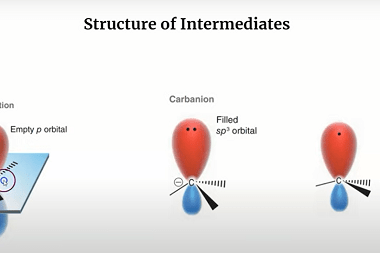
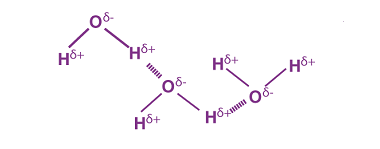
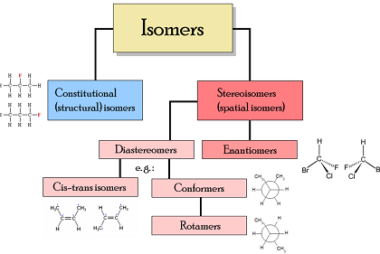
Reactions
Sure, here are some common reactions of alkanes: History of Alkanes Reactions The history of alkanes reactions dates back to the early 19th century, when chemists first began to study and understand the properties of these organic compounds. In 1825, the French chemist Michel Eugène Chevreul discovered a new substance which he named “spermaceti” from…