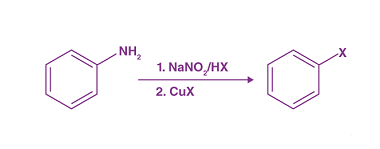
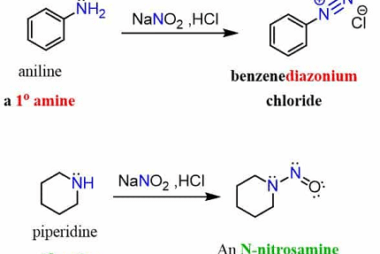
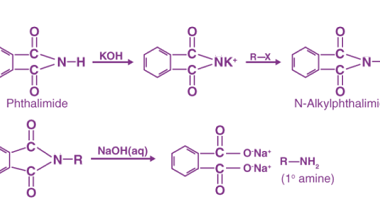
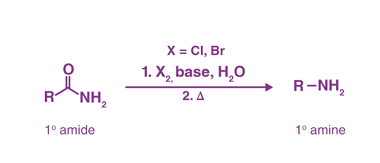Sandmeyer
The Sandmeyer reaction is a classic organic chemical reaction that is used to synthesize aryl halides from aryl diazonium salts. The reaction is named after Traugott Sandmeyer, who first reported it in 1884. Amines can be used as starting materials in the Sandmeyer reaction to synthesize aryl halides bearing amino groups. The reaction proceeds via…