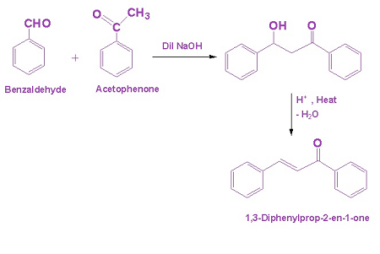
Aldol Condensation
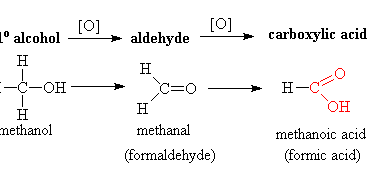
Aldol condensation is a type of organic reaction that involves the condensation of two carbonyl compounds, usually an aldehyde and a ketone, to form a β-hydroxy carbonyl compound, also known as an aldol. The word “aldol” is a combination of “aldehyde” and “alcohol”. The reaction typically requires a base catalyst, such as sodium hydroxide, to…