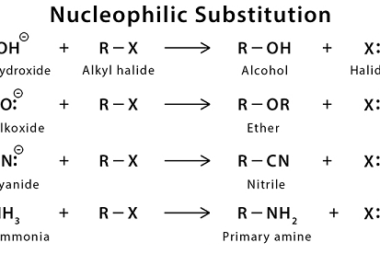
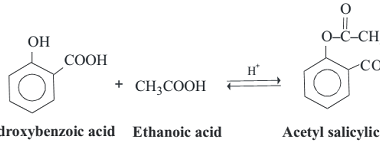
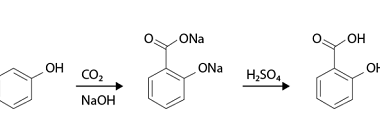
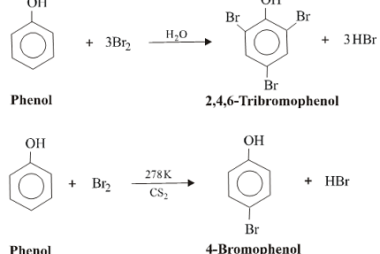
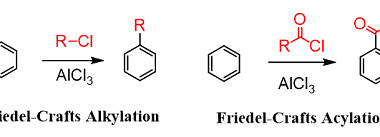
Nucleophilic substitution reactions
Nucleophilic substitution reactions are a type of organic reaction in which a nucleophile (a species with a lone pair of electrons) attacks an electrophilic center and replaces a leaving group. These reactions are typically observed in organic chemistry and are fundamental to many important organic reactions. The general mechanism for a nucleophilic substitution reaction involves…