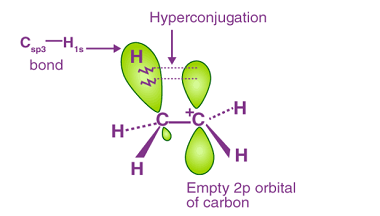
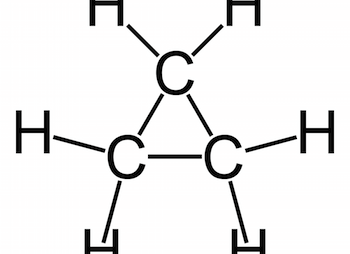
Acidity and Basicity of organic compounds
Acidity and basicity are important chemical properties of organic compounds. Acidity refers to the ability of a compound to donate a proton (H+) and form a stable conjugate base. Basicity, on the other hand, refers to the ability of a compound to accept a proton and form a stable conjugate acid. The acidity or basicity…