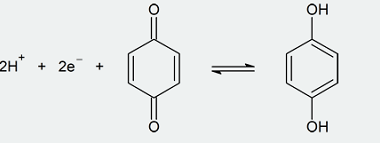
Reduction reactions of phenol
Phenol (C6H5OH) can undergo several reduction reactions, including: Overall, the reduction of phenol can lead to the formation of a range of products, depending on the choice of reducing agent and reaction conditions. History of Reduction reactions of phenol The reduction reactions of phenol have been studied and used for several decades. Here are some…