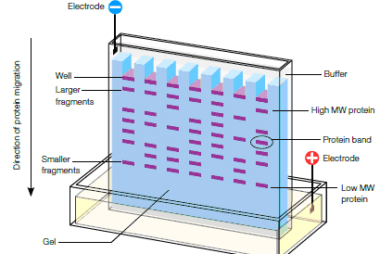
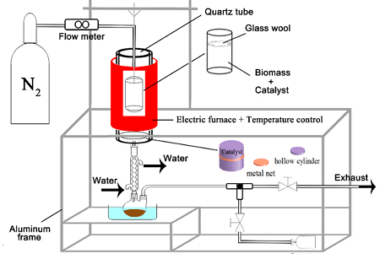
Advance Course AIIMS-SYLLABUS Chemistry syllabus Copper
Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a transition metal and belongs to Group 11 on the periodic table. Here are some key points about copper: These are some essential aspects related to copper. Its wide range of applications and unique properties make it a valuable…