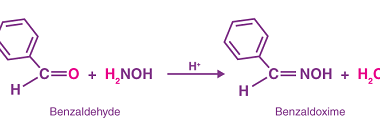
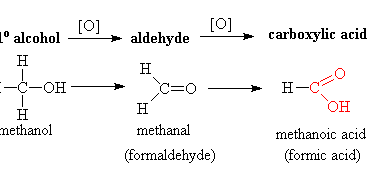
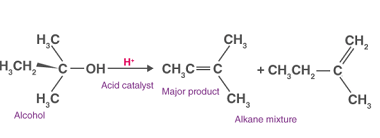
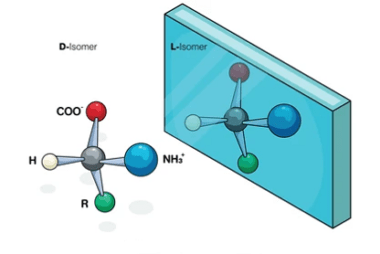
NaHSO3
NaHSO3 is the chemical formula for sodium bisulfite. It is a white crystalline powder that is soluble in water and has a slightly sulfurous odor. Sodium bisulfite is commonly used in various industries such as food processing, photography, and water treatment. In the food industry, it is used as a preservative and to prevent discoloration…