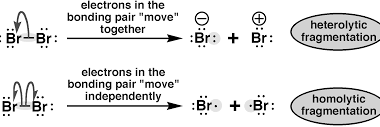
Reactive intermediates produced during homolytic and heterolytic bond cleavage
During bond cleavage, reactive intermediates can be produced as a result of either homolytic or heterolytic cleavage. In homolytic cleavage, the bond is split evenly between the two atoms, resulting in the formation of two highly reactive species called free radicals. Free radicals are atoms or molecules with an unpaired electron in their outer shell,…