Group 15 Oxides of Nitrogen and Oxoacids of phosphorus
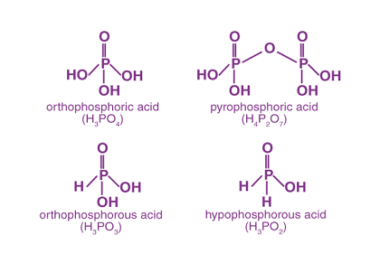
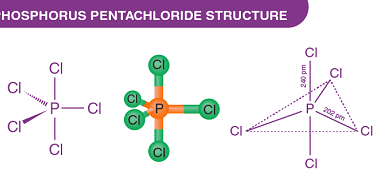
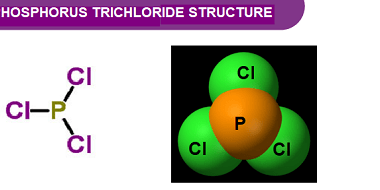
Group 15 of the periodic table consists of nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi). Both nitrogen and phosphorus form oxides and oxoacids. Oxides of Nitrogen: Oxoacids of Phosphorus: What is Required p-Block Elements Group 15 Oxides of Nitrogen and Oxoacids of phosphorus The p-block elements in Group 15 of the…