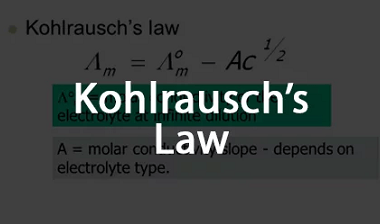
Advance Course AIIMS-SYLLABUS Chemistry syllabus Rate of a reaction
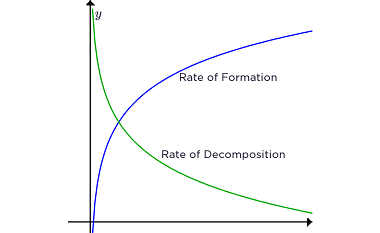
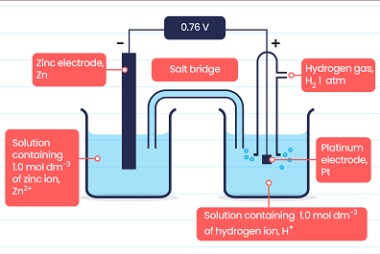
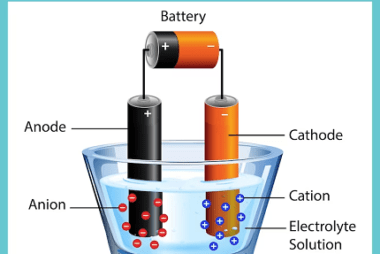
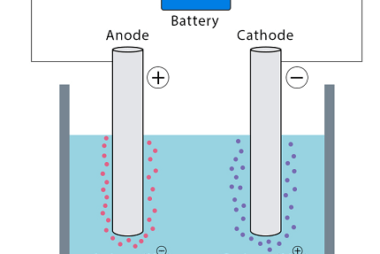
Rate of a reaction The rate of a reaction refers to how quickly a chemical reaction occurs, specifically the change in concentration of reactants or products over time. It is often expressed as the rate of disappearance of a reactant or the rate of formation of a product. The rate of a reaction can be…