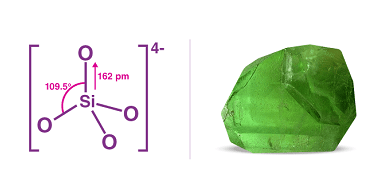
Group 14 Silicates
Group 14 silicates are a subgroup of silicate minerals that contain tetrahedral SiO4 units linked together to form a three-dimensional framework. The group includes several important minerals, including quartz, feldspar, and mica. The SiO4 tetrahedra in group 14 silicates are linked together in a way that creates a continuous network. The bonding between the tetrahedra…