Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules and the way that these arrangements affect the chemical and physical properties of those molecules.
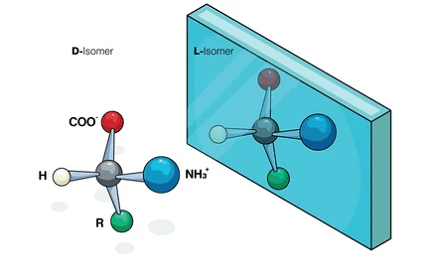
One of the most important concepts in stereochemistry is chirality, which refers to the property of a molecule that cannot be superimposed on its mirror image. Molecules that exhibit chirality are said to be chiral or asymmetric. Chirality is important in chemistry, biochemistry, and pharmacology because chiral molecules often have different biological activities and can exhibit different physical and chemical properties.
Stereochemistry also includes the study of stereoisomers, which are molecules that have the same molecular formula and connectivity but different spatial arrangements of their atoms. Stereoisomers can be further classified as enantiomers, diastereomers, and geometric isomers based on the specific type of difference in their spatial arrangements.
Enantiomers are stereoisomers that are mirror images of each other and cannot be superimposed. They have identical physical and chemical properties, except for their interaction with other chiral molecules, such as enzymes or receptors. This property makes enantiomers important in fields such as drug design, where one enantiomer can be more effective or have fewer side effects than the other.
Diastereomers are stereoisomers that are not mirror images of each other and have different physical and chemical properties. They arise when a molecule has two or more stereocenters (atoms bonded to four different groups), and their arrangement in space is different.
Geometric isomers are stereoisomers that have the same connectivity but different arrangements of groups around a double bond or a ring. They are named cis-trans isomers or E-Z isomers based on their relative orientation.
Understanding stereochemistry is crucial for chemists, biochemists, and pharmacologists because it can have significant effects on the behavior of molecules, especially in biological systems.
What is Required Alkyl Halides Their stereochemical aspects
Required alkyl halides are organic compounds that contain a halogen atom (fluorine, chlorine, bromine, or iodine) bonded to a carbon atom, which is also bonded to an alkyl group. Stereochemical aspects of alkyl halides are important in determining their physical and chemical properties, as well as their reactivity in chemical reactions.
One of the most important stereochemical aspects of alkyl halides is their chirality. If the carbon atom to which the halogen is attached is a stereocenter, the alkyl halide can exist as two enantiomers, which are mirror images of each other and cannot be superimposed. This is because the carbon atom has four different groups bonded to it, and the spatial arrangement of these groups determines the molecule’s chirality.
The stereochemistry of alkyl halides can also affect their reactivity in chemical reactions. For example, in nucleophilic substitution reactions, the stereochemistry of the alkyl halide can determine whether the reaction proceeds via an SN1 or an SN2 mechanism. SN1 reactions are favored by alkyl halides that have carbocations as intermediates, which are more stable when the carbon atom to which the halogen is attached is tertiary. SN2 reactions, on the other hand, are favored by alkyl halides that have less steric hindrance, which allows the nucleophile to attack the carbon atom to which the halogen is attached more easily.
In addition, the stereochemistry of alkyl halides can affect their solubility, boiling point, and other physical properties. For example, enantiomers of chiral alkyl halides can have different melting points, boiling points, and optical rotations, depending on the specific arrangement of their atoms in space.
Overall, the stereochemical aspects of alkyl halides are important in determining their physical and chemical properties, as well as their reactivity in chemical reactions.
History of Alkyl Halides Their stereochemical aspects
The history of alkyl halides and their stereochemical aspects dates back to the mid-19th century, when organic chemistry was just beginning to emerge as a distinct field of study. In 1850, the German chemist August Wilhelm von Hofmann discovered that when ethyl iodide was treated with silver nitrate, it produced two isomeric compounds with different physical and chemical properties. This was the first example of a stereoisomer of an alkyl halide, and it provided early evidence for the importance of stereochemistry in organic chemistry.
In the following decades, other chemists, such as Charles Adolphe Wurtz and Louis Pasteur, made important contributions to the study of alkyl halides and their stereochemistry. Wurtz discovered that alkyl halides could be synthesized by reacting alcohols with hydrogen halides, which allowed for the preparation of a wide range of alkyl halides for study. Pasteur, on the other hand, showed that certain organic compounds, including some alkyl halides, could exist as two enantiomers, or mirror-image isomers, which could have different physical and chemical properties.
In the early 20th century, the development of X-ray crystallography allowed chemists to determine the three-dimensional structures of molecules, including alkyl halides, with increasing accuracy. This led to a deeper understanding of the stereochemical aspects of alkyl halides and their importance in chemical reactions.
In the decades that followed, researchers continued to study alkyl halides and their stereochemistry, discovering new reactions and mechanisms, as well as developing new synthetic methods and applications. Today, alkyl halides continue to be an important class of compounds in organic chemistry, with applications in fields such as pharmaceuticals, materials science, and biotechnology.
Structures of Alkyl Halides Their stereochemical aspects
The structure of an alkyl halide is characterized by the presence of a halogen atom (fluorine, chlorine, bromine, or iodine) attached to a carbon atom, which is also bonded to one or more alkyl groups. The carbon atom to which the halogen is attached can be a stereocenter, meaning it has four different groups attached to it, which results in the possibility of the alkyl halide existing as two enantiomers.
The stereochemistry of alkyl halides is important in determining their physical and chemical properties, as well as their reactivity in chemical reactions. For example, the stereochemistry of alkyl halides can determine whether the reaction proceeds via an SN1 or an SN2 mechanism in nucleophilic substitution reactions. The stereochemistry of alkyl halides can also affect their solubility, boiling point, and other physical properties.
The structure of an alkyl halide can be represented using various methods, including Lewis structures, condensed structural formulas, and line-angle structures. In a Lewis structure, the halogen atom is shown as a single bond to the carbon atom, with the other valence electrons on the halogen represented as lone pairs. Condensed structural formulas and line-angle structures are more commonly used in organic chemistry, and they show the carbon atom and the attached alkyl groups as lines, with the halogen atom indicated by its symbol.
Examples of alkyl halides and their structures include:
- Ethyl chloride (CH3CH2Cl)
- Isopropyl bromide (CH3)2CHBr
- tert-Butyl iodide ((CH3)3C-I)
- Allyl fluoride (CH2=CHCH2F)
Overall, the structure of alkyl halides and their stereochemistry play a critical role in understanding their properties and behavior in chemical reactions.
Nomenclature of Alkyl Halides Their stereochemical aspects
The nomenclature of alkyl halides follows the guidelines of the International Union of Pure and Applied Chemistry (IUPAC) naming system. The basic principle of naming alkyl halides is to identify the longest carbon chain that contains the halogen atom and to indicate the position of the halogen atom with a prefix or a suffix. If the carbon chain contains multiple halogens, each halogen is numbered and named separately.
The position of the halogen atom is indicated by a prefix, such as chloro-, bromo-, iodo-, or fluoro-, or by a suffix, such as -chloride, -bromide, -iodide, or -fluoride. The prefix or suffix is placed in front of the name of the alkyl group, and the two parts are separated by a hyphen. The alkyl groups are named based on the number of carbon atoms in the longest continuous chain that contains the halogen atom, with the prefix indicating the number of carbon atoms (e.g., methyl-, ethyl-, propyl-, butyl-).
If the alkyl halide contains a double or triple bond, the position of the halogen atom is indicated by a number, starting from the end of the chain nearest the double or triple bond. If the molecule contains more than one halogen atom, the positions of each halogen atom are indicated by a number, with the halogens listed in alphabetical order.
Examples of alkyl halide names using IUPAC nomenclature include:
- Chloromethane (CH3Cl)
- 2-Bromo-3-methylpentane (CH3CH2CH(Br)CH(CH3)CH2CH3)
- 1-Iodo-4-fluorocyclohexane (C6H11I-F)
- 3-Chlorocyclobutanecarboxylic acid (C4H7ClO2)
Overall, the nomenclature of alkyl halides follows a standardized system that allows for clear and unambiguous identification of the compound’s structure and composition.
Production of Alkyl Halides Their stereochemical aspects
Alkyl halides can be produced by several methods, including halogenation of alkanes, addition of hydrogen halides to alkenes, and substitution of a hydroxyl group with a halogen atom in alcohols. The stereochemistry of the resulting alkyl halide depends on the method used and the configuration of the starting material.
- Halogenation of alkanes: Alkanes can be chlorinated or brominated by exposure to chlorine or bromine in the presence of a catalyst, such as iron or aluminum chloride. The stereochemistry of the resulting alkyl halide depends on the arrangement of the hydrogen atoms on the carbon atom being halogenated. If the carbon atom is a stereocenter, the product can exist as two enantiomers.
- Addition of hydrogen halides to alkenes: Alkenes can be reacted with hydrogen halides, such as hydrogen chloride or hydrogen bromide, to form alkyl halides. The stereochemistry of the resulting alkyl halide depends on the stereochemistry of the starting material. If the alkene is a stereocenter, the product can exist as two enantiomers.
- Substitution of a hydroxyl group with a halogen atom in alcohols: Alcohols can be converted into alkyl halides by substitution of a hydroxyl group with a halogen atom, typically chlorine or bromine, in the presence of a strong acid, such as hydrochloric acid or hydrobromic acid. The stereochemistry of the resulting alkyl halide depends on the configuration of the starting material. If the carbon atom to which the hydroxyl group is attached is a stereocenter, the product can exist as two enantiomers.
Overall, the stereochemistry of alkyl halides depends on the method of production and the stereochemistry of the starting material. Alkyl halides with stereocenters can exist as two enantiomers, which can have different physical and chemical properties and can react differently in chemical reactions.
Case Study on Alkyl Halides Their stereochemical aspects
One interesting case study involving alkyl halides and their stereochemical aspects is the synthesis of the anti-inflammatory drug naproxen. Naproxen is a nonsteroidal anti-inflammatory drug (NSAID) used to relieve pain and inflammation, and it is marketed under several trade names, including Aleve and Naprosyn.
The synthesis of naproxen involves the conversion of a carboxylic acid group into an alkyl halide, followed by a series of chemical reactions to create the final product. The stereochemistry of the alkyl halide intermediate is critical to the overall synthesis and the final product’s efficacy.
In the first step of the synthesis, the carboxylic acid group in a chiral starting material, (S)-(+)-2-(6-methoxy-2-naphthyl)propionic acid, is converted into the corresponding acid chloride by reaction with thionyl chloride. The acid chloride is then reacted with the chiral amine (S)-(-)-α-methylbenzylamine in the presence of a base, such as triethylamine, to form an intermediate amide.
The amide is then treated with phosphoryl chloride, which converts the amide into an imidoyl chloride intermediate. The imidoyl chloride is reacted with a chiral alcohol, (S)-(-)-1-phenylethanol, in the presence of a base to form an alkyl halide intermediate. The stereochemistry of the alkyl halide intermediate is critical to the synthesis’s success, as it determines the stereochemistry of the final product.
In the final steps of the synthesis, the alkyl halide intermediate is reacted with sodium hydride and carbon dioxide to form an intermediate carboxylic acid, which is then converted into naproxen by reaction with a base, such as sodium hydroxide. The final product is obtained as a single enantiomer, with the stereochemistry determined by the configuration of the chiral starting material and the stereochemistry of the alkyl halide intermediate.
This case study illustrates the importance of stereochemistry in the synthesis of drugs and other complex organic molecules. The ability to control and manipulate stereochemistry is critical to the development of effective pharmaceuticals, as different stereoisomers can have different biological activity, toxicity, and pharmacokinetic properties.
White paper on Alkyl Halides Their stereochemical aspects
Introduction:
Alkyl halides, also known as haloalkanes, are organic compounds that contain one or more halogen atoms, such as chlorine, bromine, or iodine, bonded to a carbon atom in an alkyl group. The stereochemistry of alkyl halides is an important aspect of their properties and reactivity. This white paper will discuss the stereochemical aspects of alkyl halides, including their structures, nomenclature, and production methods.
Structures of Alkyl Halides:
Alkyl halides can exist in different stereoisomeric forms, depending on the arrangement of atoms around the carbon atom to which the halogen atom is bonded. The two main types of stereoisomers are enantiomers and diastereomers. Enantiomers are mirror images of each other and cannot be superimposed, while diastereomers are non-mirror image stereoisomers.
Alkyl halides can also have chiral centers, which are carbon atoms bonded to four different groups. The stereochemistry of the chiral center can be described using the R/S system or the D/L system, depending on the configuration of the groups around the chiral center.
Nomenclature of Alkyl Halides:
The nomenclature of alkyl halides follows the same rules as for other organic compounds, with the halogen atom named as a substituent. The position of the halogen atom is indicated by a number, and the stereochemistry of the chiral center, if present, is indicated by the R/S or D/L system.
Production of Alkyl Halides:
Alkyl halides can be produced by several methods, including halogenation of alkanes, addition of hydrogen halides to alkenes, and substitution of a hydroxyl group with a halogen atom in alcohols. The stereochemistry of the resulting alkyl halide depends on the method used and the configuration of the starting material.
Halogenation of alkanes involves the reaction of an alkane with a halogen, such as chlorine or bromine, in the presence of a catalyst, such as iron or aluminum chloride. The stereochemistry of the resulting alkyl halide depends on the arrangement of the hydrogen atoms on the carbon atom being halogenated. If the carbon atom is a stereocenter, the product can exist as two enantiomers.
Addition of hydrogen halides to alkenes involves the reaction of an alkene with a hydrogen halide, such as hydrogen chloride or hydrogen bromide. The stereochemistry of the resulting alkyl halide depends on the stereochemistry of the starting material. If the alkene is a stereocenter, the product can exist as two enantiomers.
Substitution of a hydroxyl group with a halogen atom in alcohols involves the reaction of an alcohol with a halogen, typically chlorine or bromine, in the presence of a strong acid, such as hydrochloric acid or hydrobromic acid. The stereochemistry of the resulting alkyl halide depends on the configuration of the starting material. If the carbon atom to which the hydroxyl group is attached is a stereocenter, the product can exist as two enantiomers.
Conclusion:
In conclusion, the stereochemistry of alkyl halides is an essential aspect of their properties and reactivity. Alkyl halides can exist in different stereoisomeric forms, including enantiomers and diastereomers, depending on the arrangement of atoms around the carbon atom to which the halogen atom is bonded. The stereochemistry of chiral centers in alkyl halides can be described using the R/S or D/L system, and the nomenclature of alkyl halides follows the same rules as for other organic compounds. The stereochemistry of the starting material can have a significant impact on the stereochemistry of the final product, and various methods can be used to produce alkyl halides, including halogenation of alkanes, addition of hydrogen halides to alkenes, and substitution of a hydroxyl group with a halogen atom in alcohols. Understanding the stereochemical aspects of alkyl halides is crucial in designing and predicting their properties and reactivity, making it an essential topic in organic chemistry.
