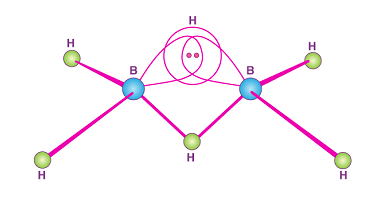
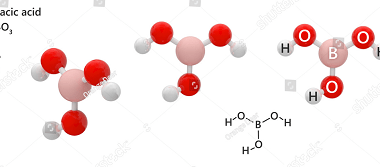
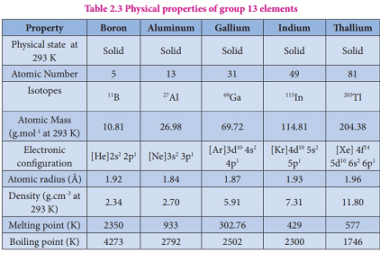
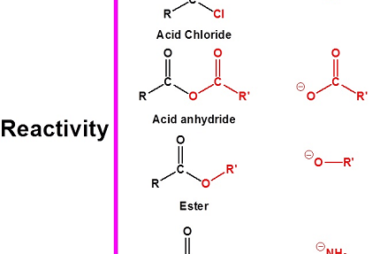
Group 13 Aluminium chloride
Aluminium chloride is a chemical compound with the formula AlCl3. It is a white to pale yellow powder or granular solid, and it is highly soluble in water. Aluminium chloride is commonly used as a Lewis acid catalyst in organic chemistry reactions. Group 13 refers to the group of elements in the periodic table that…