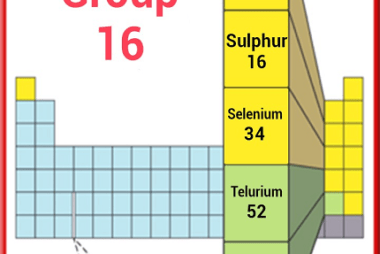
Group 16 Preparation/Manufacture
Group 16 of the periodic table consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po). Here’s a brief overview of the preparation/manufacture of these elements: Oxygen (O): Oxygen is the most abundant element on Earth and can be found in the atmosphere as well as in various minerals and…